MAIN PROJECTS:
i) The role of non-coding RNAs (microRNAs/lncRNAs) in BCR-signalling and microenvironmental interactions in B cell malignancies (Chronic Lymphocytic Leukemia, B-Cell Lymphomas)
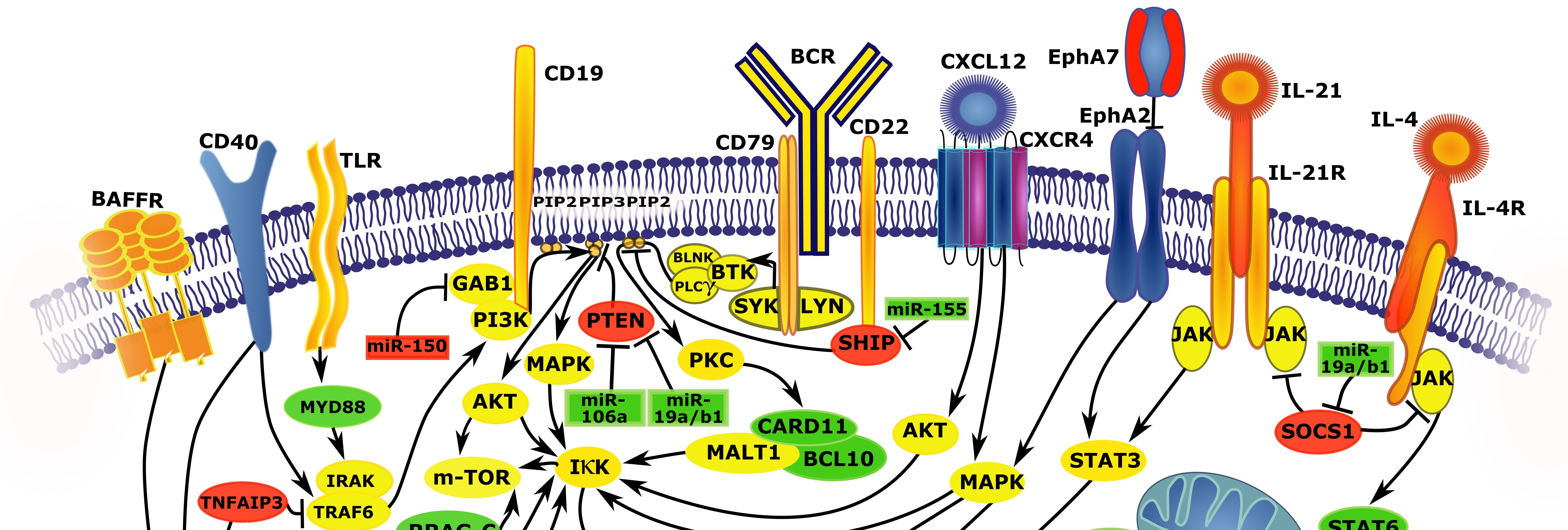
ii) The regulation of BCR signalling
iii) The regulation and function of CD20
iv) Development of novel targeted therapies (miR-therapy, targeting of BCR-signalling/adhesion)
v) Prognostic and predictive markers in hematological malignancies
VIDEO TOUR THROUGH OUR LAB AND FACILITIES
i-iii) MICROENVIRONMENTAL INTERACTIONS IN NORMAL AND MALIGNANT IMMUNE CELLS. The microenvironment of immune niches plays an important role in the onset, progression, and resistance of hematological malignancies. We largely focus on the role of non-coding RNAs (especially microRNAs [miRNAs] and long non-coding RNAS [lncRNAs]) in these pathways (Sharma et al, Blood, 2020; Musilova et al, Blood, 2018; Mraz et al, Blood, 2014; Musilova and Mraz, Leukemia, 2015). The discovery of non-coding RNAs, particularly miRNAs, changed the understanding of the biology of normal and malignant B cell and regulation of cell physiology in general. MicroRNAs often participate in positive/negative feedback/feed-forward regulatory loops that coordinate microenvironmental interactions of B cells (see Musilova and Mraz, Leukemia, 2015; Sharma et al, Blood, 2020; Mraz et al, Blood, 2014; Cerna et al, Leukemia, 2019). Our data demonstrate that microRNAs (miRNAs) play a key role in the regulation of microenvironmental interactions. We also study the regulation and function of a cell-surface molecule CD20 in this context, since this molecule is used as a therapeutic target for over 20 years, but its regulation and function is unclear (Pavlasova and Mraz, Haematologica, 2020; Pavlasova et al, Blood, 2016; Pavlasova et al, Leukemia, 2018).
iv) DEVELOPMENT OF NOVEL TARGETED THERAPIES. The approval of drugs that target the B cell receptor pathway (BCR) was a milestone in the therapy of B cell malignancies. However, the use of these drugs does not lead to a cure and patients relapse. We aim to i) describe the molecular pathways affected by BCR inhibition and understand mechanisms that leukemic cells use to survive, and ii) to define and test druggable therapeutic targets that should be therapeutically combined with BTK/PI3K inhibition (Seda et al, Blood, 2021; Pavlasova et al, Blood, 2016; Sharma et al, Blood, 2020; patent US 11648255B2).
v) PROGNOSTIC AND PREDICTIVE BIOMARKERS. We have for the first time established a link between DNA damage response and the regulation of B cell receptor signalling in B cells via microRNAs (Cerna et al, Leukemia, 2019), and this miRNA can be used as a robust biomarker of response to chemoimmunotherapy (2 patents). We have also shown that miR-150 can be used as a predictor of early relapse or death in follicular lymphoma (Musilova et al, Blood, 2018; patent pending).
FURTHER INFORMATION
The biology of malignancies derived from "mature" B cells is largely driven by two molecular pathways that are crucial for the biology of B cells (i) deregulation of B cell receptor signalling, (ii) microenvironmental signals in lymph nodes and bone marrow such as B-T cell interactions (reviewed in Seda and Mraz, EJH, 2014). These events are interconnected since the normal and malignant B cells are stimulated by antigens and antigen-presenting cells in the context of their microenvironment, and B cells engage in interactions with supprotive stromal cells and T cells. Activation of both these pathways leads to up-regulation of numerous anti-apoptotic/pro-proliferative molecules and enhances receptiveness to BCR-signalling and vice versa. It has been shown that microenvirment miches are both important in the onset, progression and resistance of B cell malignancies like chronic lymphocytic leukemia (CLL), follicular lymphoma (FL), diffuse large B cell lymphoma (DLBCL), and mantel cell lymphoma (MCL). Targeting microenvironmental interactions in immune niches is currently one of the most promising therapeutic approaches and an important part of the mechanism of action of BCR-inhibitors like ibrutinib or idelalisib. However, the mechanisms for BCR (de)regulation and mechanism of action of BCR-inhibitors are largely unknown (reviewed in Seda and Mraz, EJH, 2014; Ondrisova and Mraz, Fron Oncology, 2020). Similarly, the role of miRNAs in the regulation of the complex BCR signalling cascade remains one of the highly interesting, yet poorly explored questions, in the biology of normal and malignant B cells.
FUNDING:
Private individuals : Iva Holubova, Pavel Holub, Martin C. Smehlik, David B. Smehlik (in the amount of 6.000 CZK).
GACR: Czech Science Foundation, EXPRO grant.
ERC GRANT: European Research Council
NICR: National Institute for Cancer Research
AZV MZ CR : grant agency of the Ministry of Health of the Czech Republic
EHA : European Hematology Association
JCMM/JMK : South Moravian Region