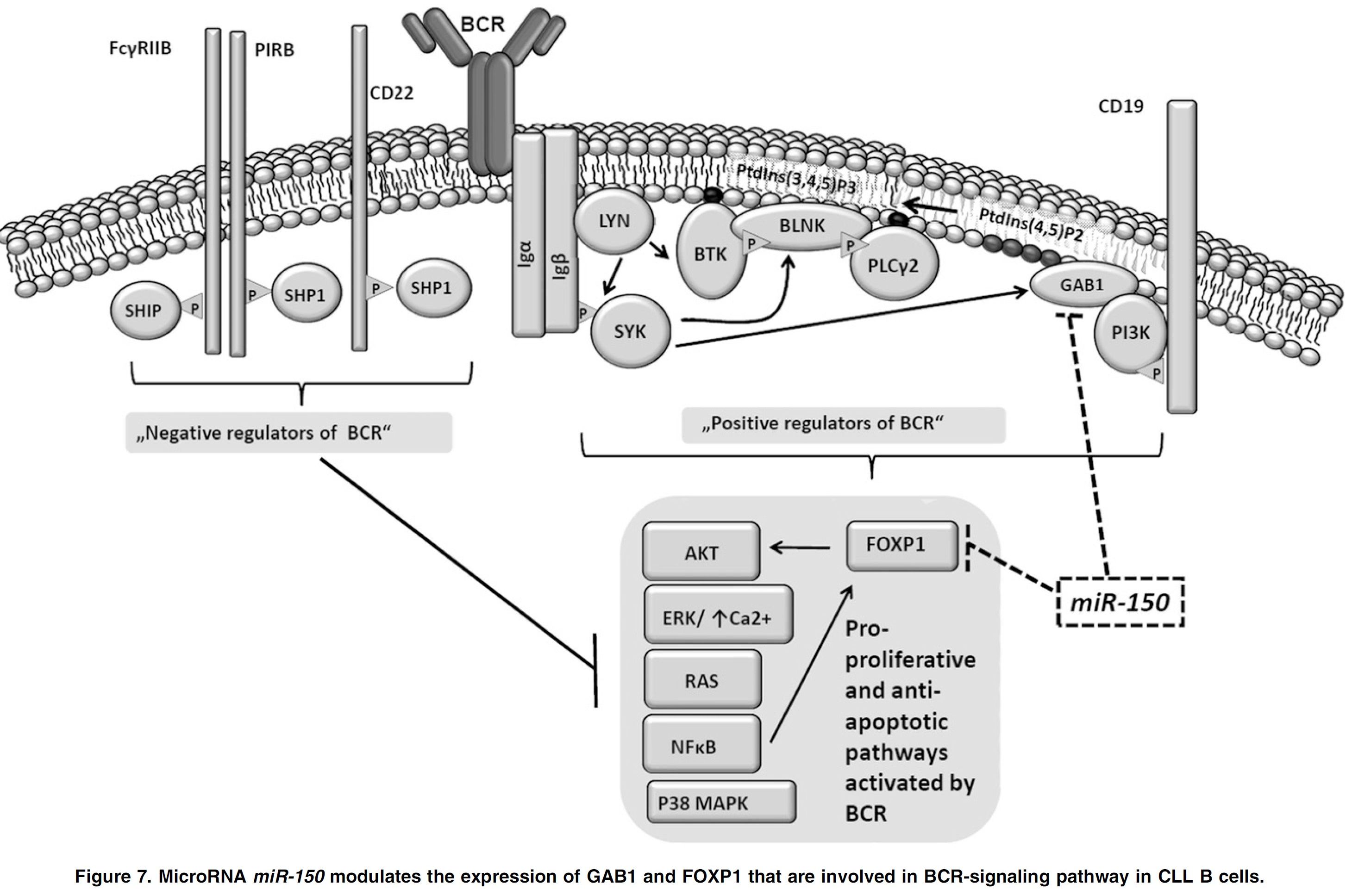
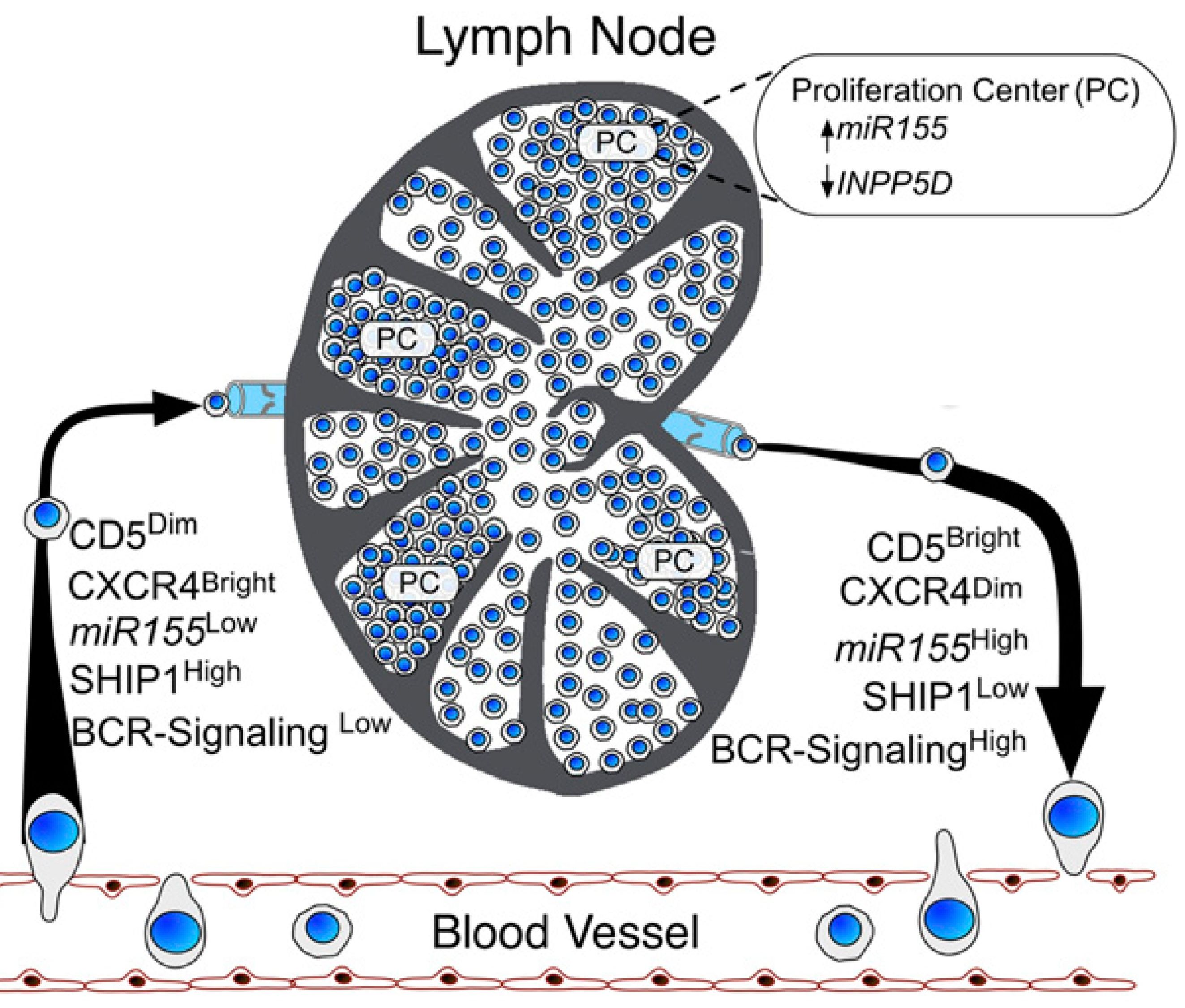
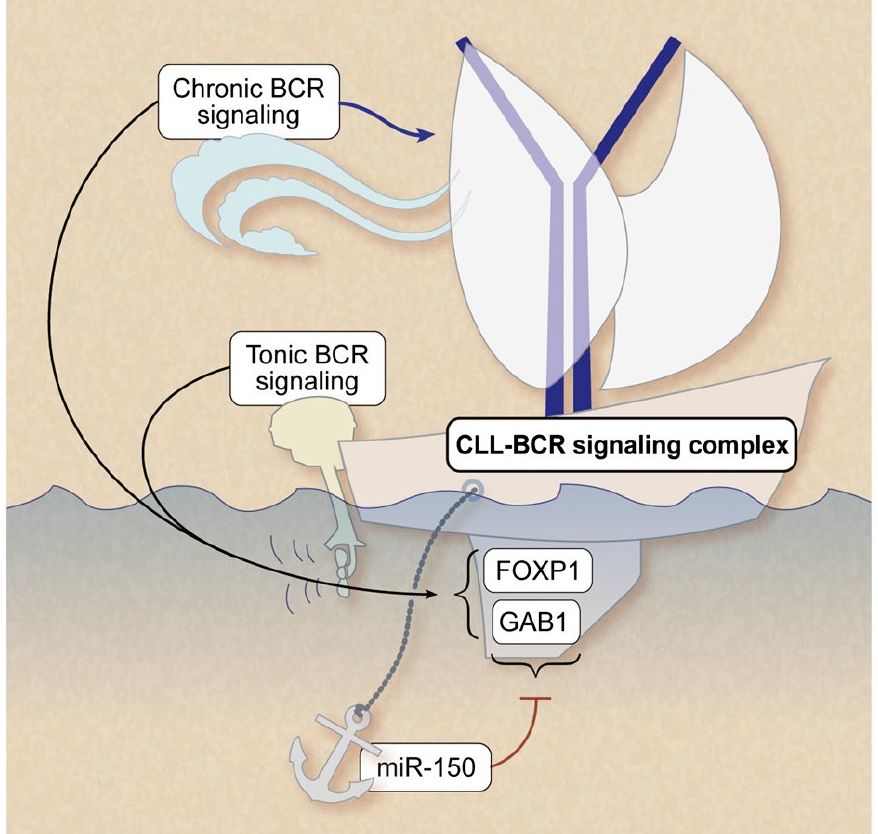
LEUKEMIA. 2026 Feb (epub). IF=13,4
Repression of miR-29 via MYC Leads to Increased CD40 Signaling in Transformed Follicular Lymphoma.
Filip D, Litzmanova K, Michaelou A, Kledus F, Devan J, Boudny M, Hoferkova E, Sharma S, Seda V, Zeni P, Borsky M, Matulova K, Kren L, Oppelt J, Blavet N, Hejret V, Urik M, Mareckova A, Rimsza L, Kamaradova K, Belada D, Sykorova A, Mocikova H, Trneny M, Prouzova Z, Evans A, Danilov A, Horn H, Ott G, Staber P, Mayer J, Friedberg J, Janikova A,
Mraz M
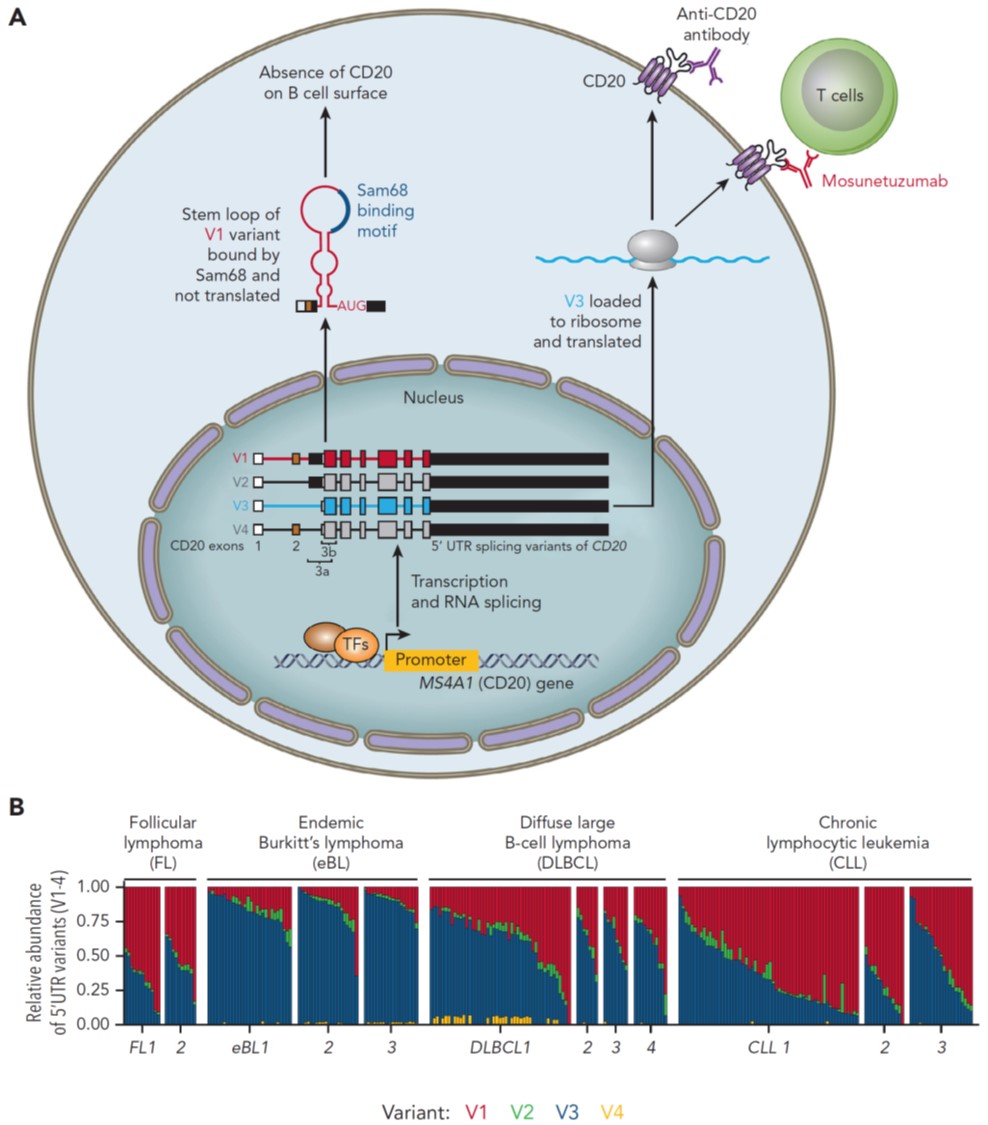
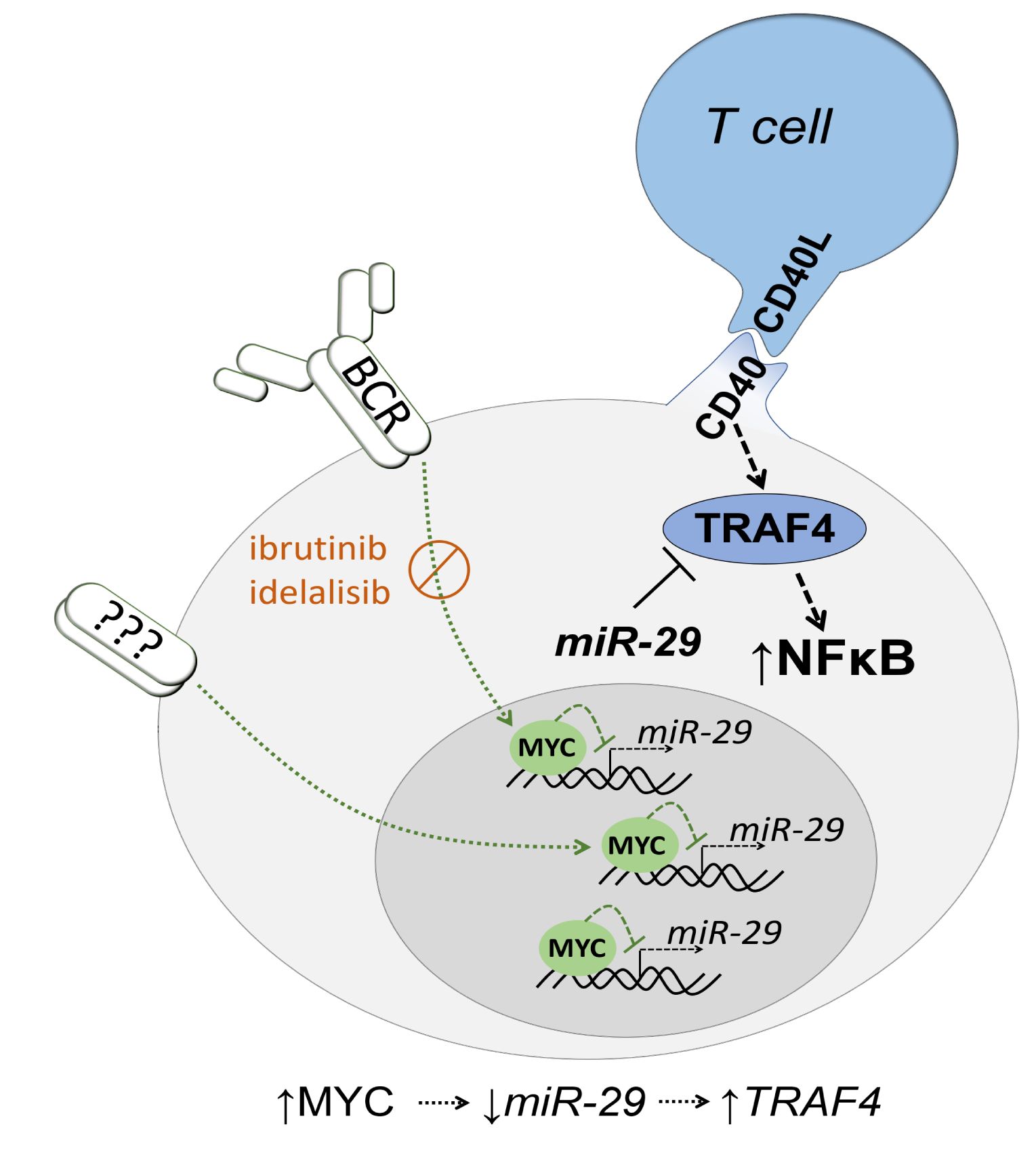
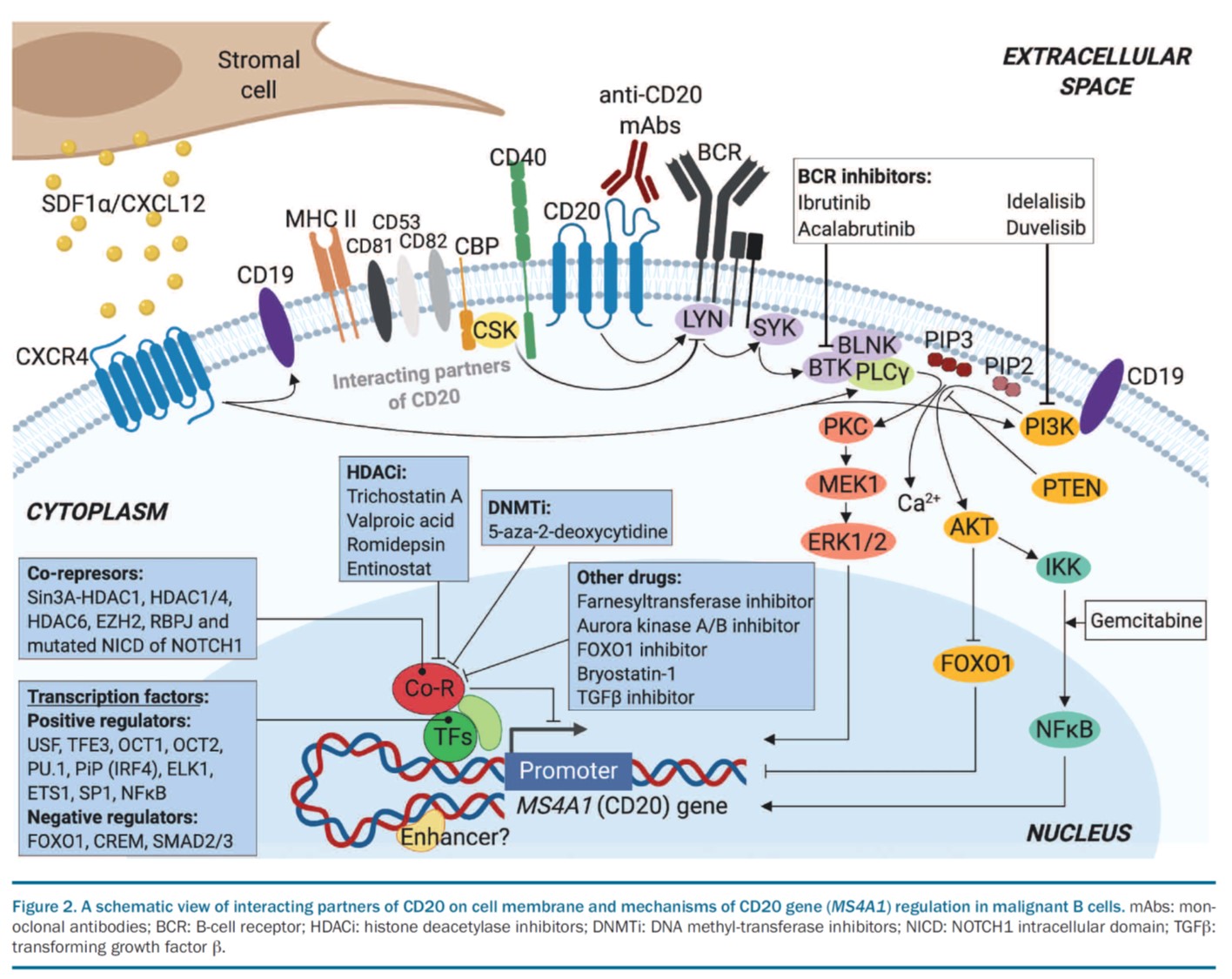
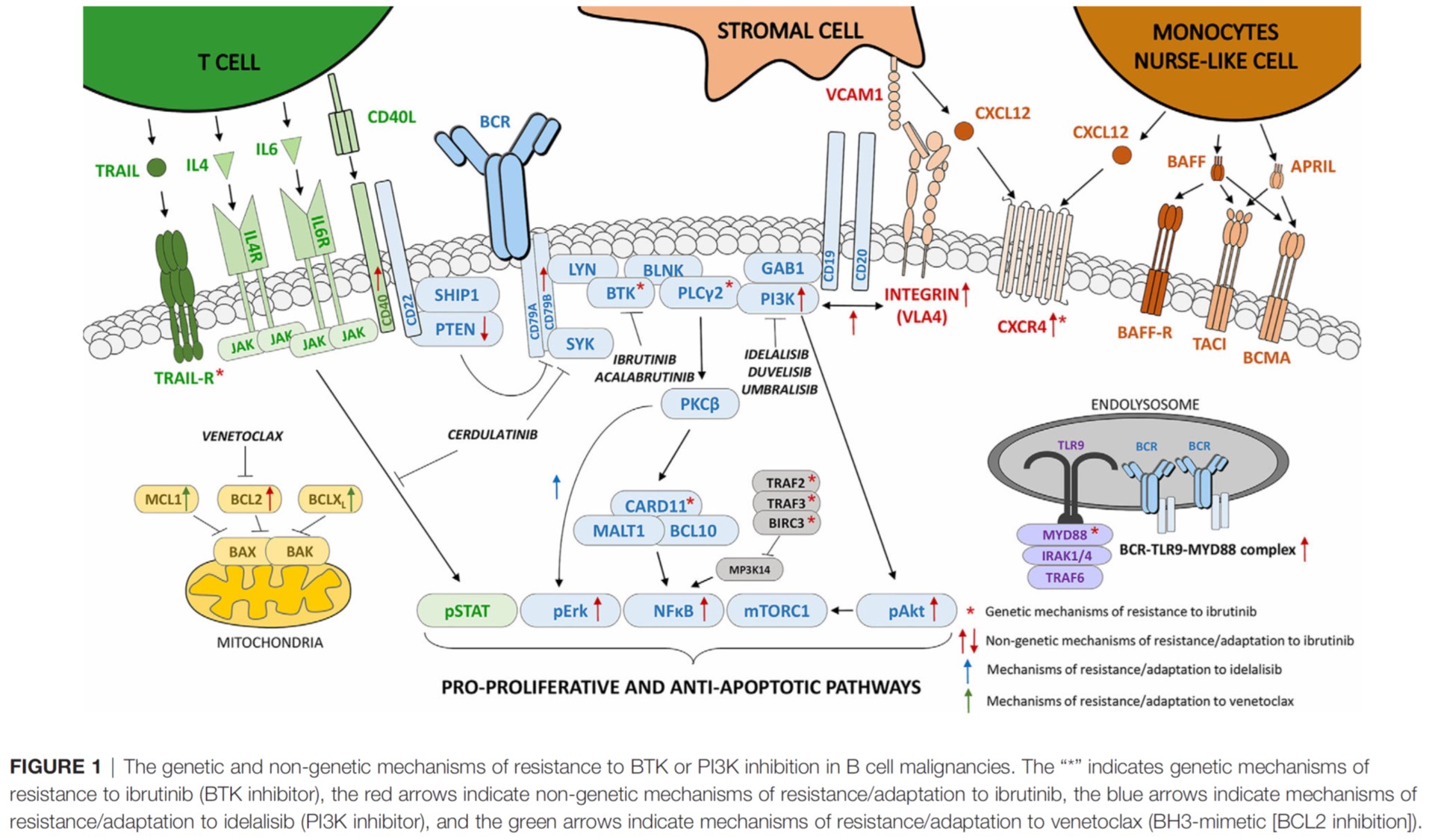
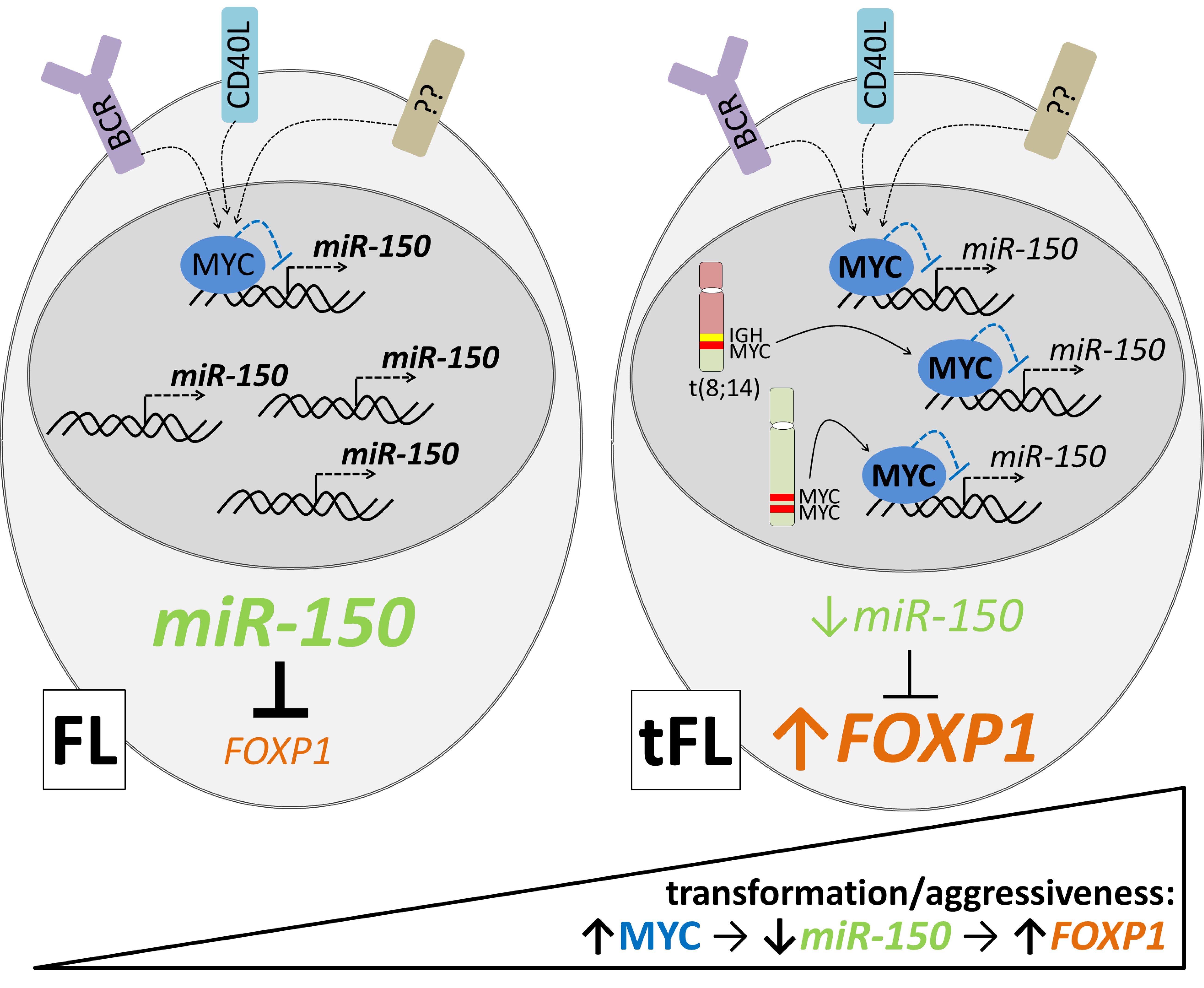
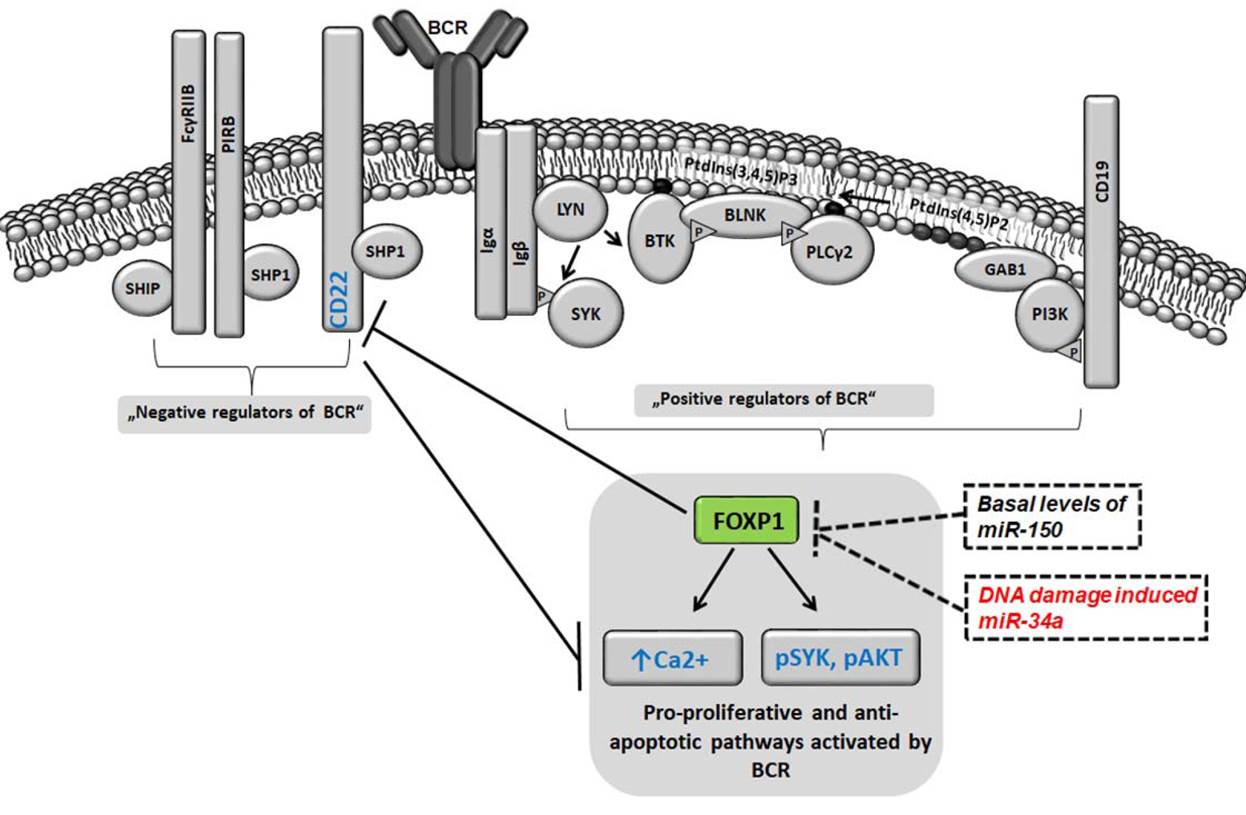
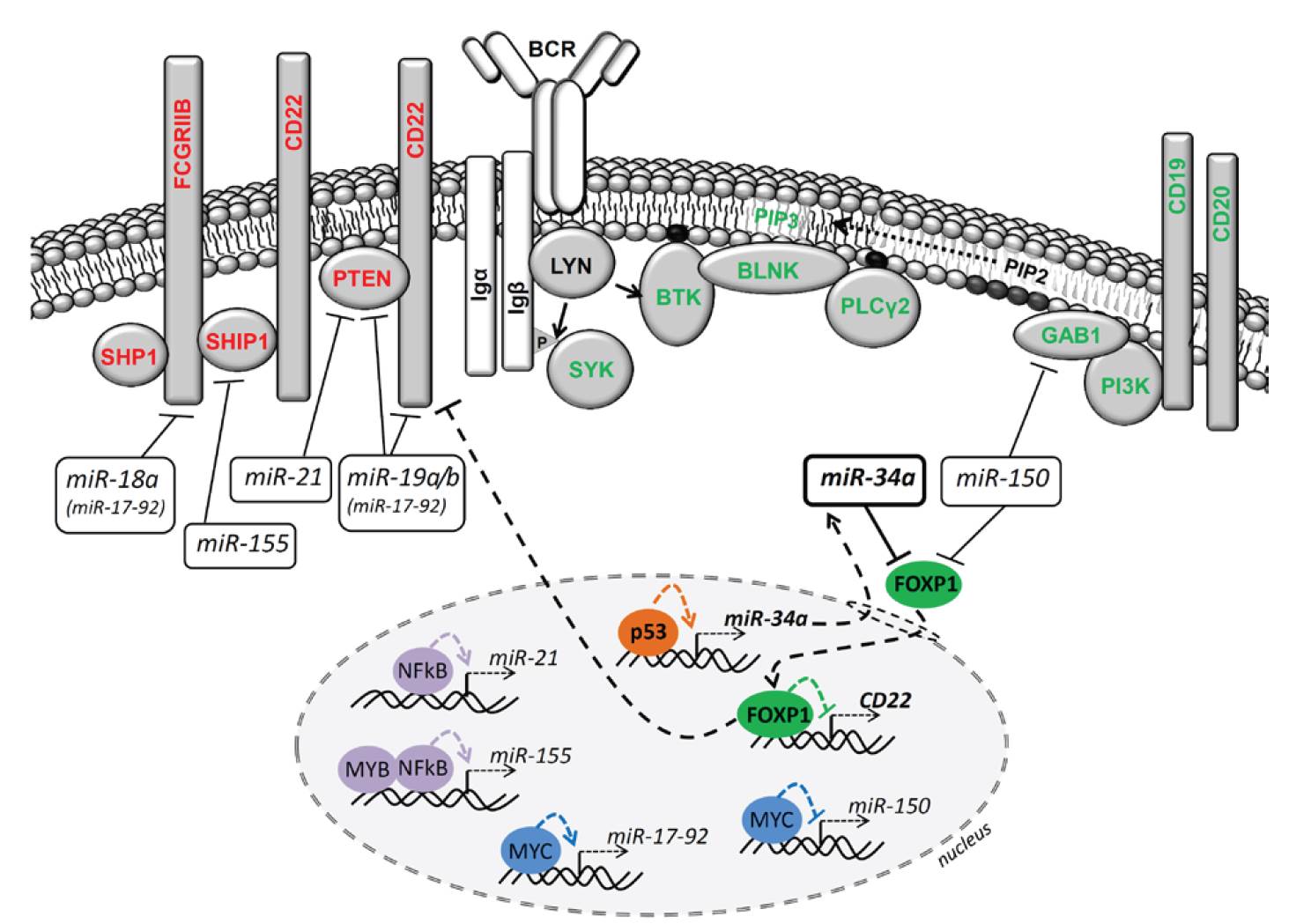
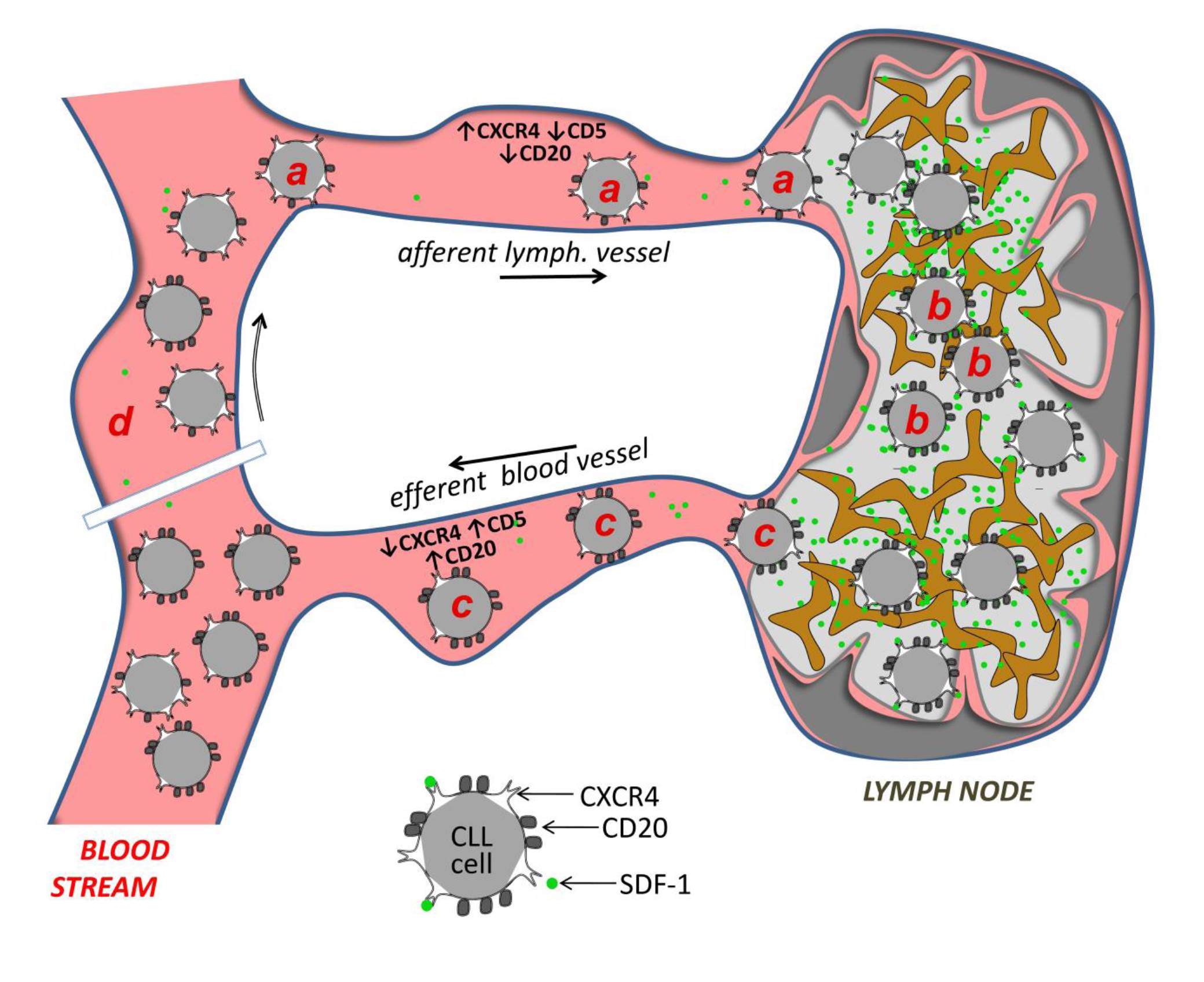
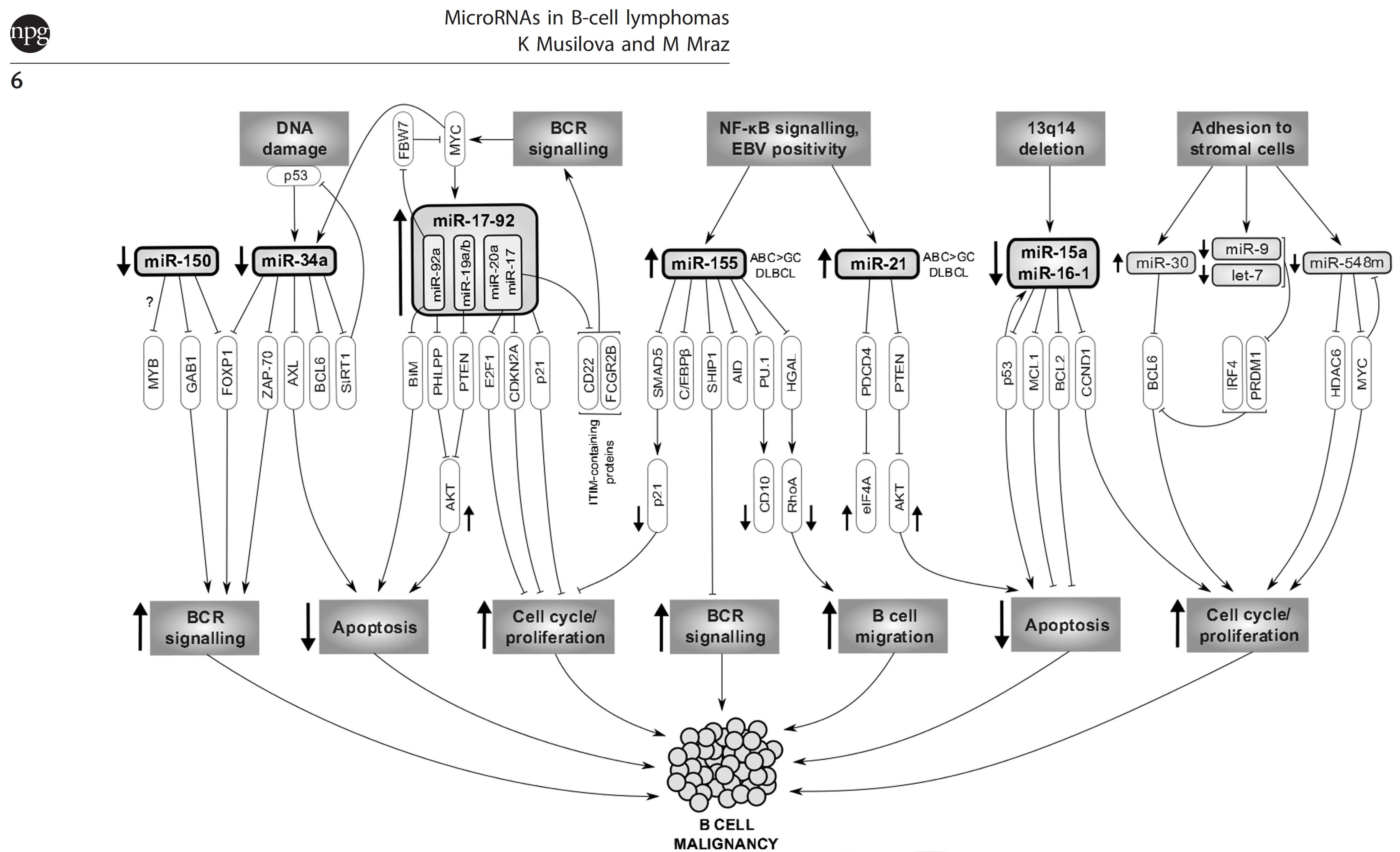
Follicular lymphoma (FL) patients are at risk of transformation to aggressive high-grade lymphoma (tFL). Although several genetic alterations have been implicated in transformation, the contribution of microenvironmental interactions and post-transcriptional regulation by non-coding RNAs remains poorly defined. We performed the first matched profiling of mRNAs and short non-coding RNAs (miRNAs) in paired FL and tFL samples (n = 11 pairs), identifying 1,075 differentially expressed mRNAs and 19 miRNAs, including repression of the miR-29 family (miR-29a/b/c) in tFL. Mechanistically, MYC directly represses miR-29 transcription in tFL, leading to upregulation of its target TRAF4, which enhances CD40 signaling and supports malignant B-cell proliferation. CD40 pathway activity was increased in 90% of tFL cases and contrasted with reduced T-cell numbers in tFL niches, suggesting that the MYC-miR-29-TRAF4 axis and heightened CD40 signaling represent an adaptation to diminished CD40L T-cell support. Clinically, low expression of all miR-29 family members was associated with shorter overall and progression-free survival in FL (n = 185), including in multivariate analysis, and low miR-29c predicted inferior overall survival in an independent validation cohort (n = 92) from the first-line R-CHOP trial (SWOG S0016, NCT00006721).