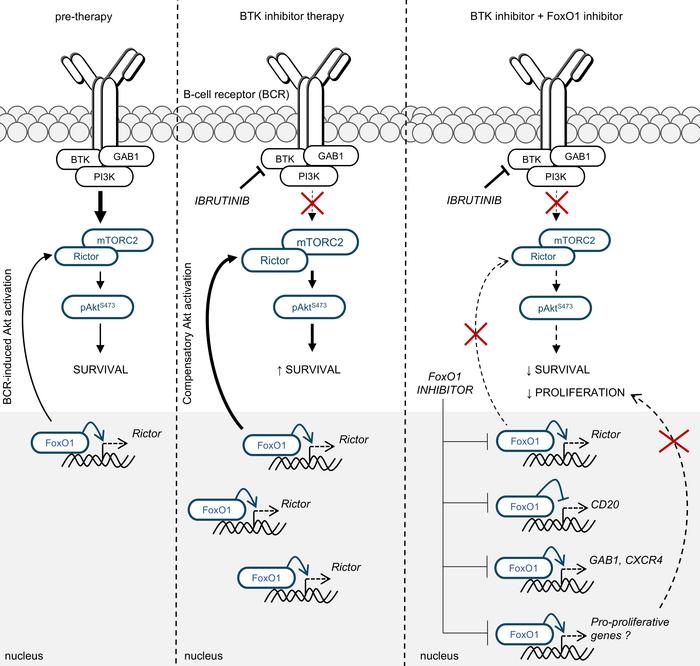
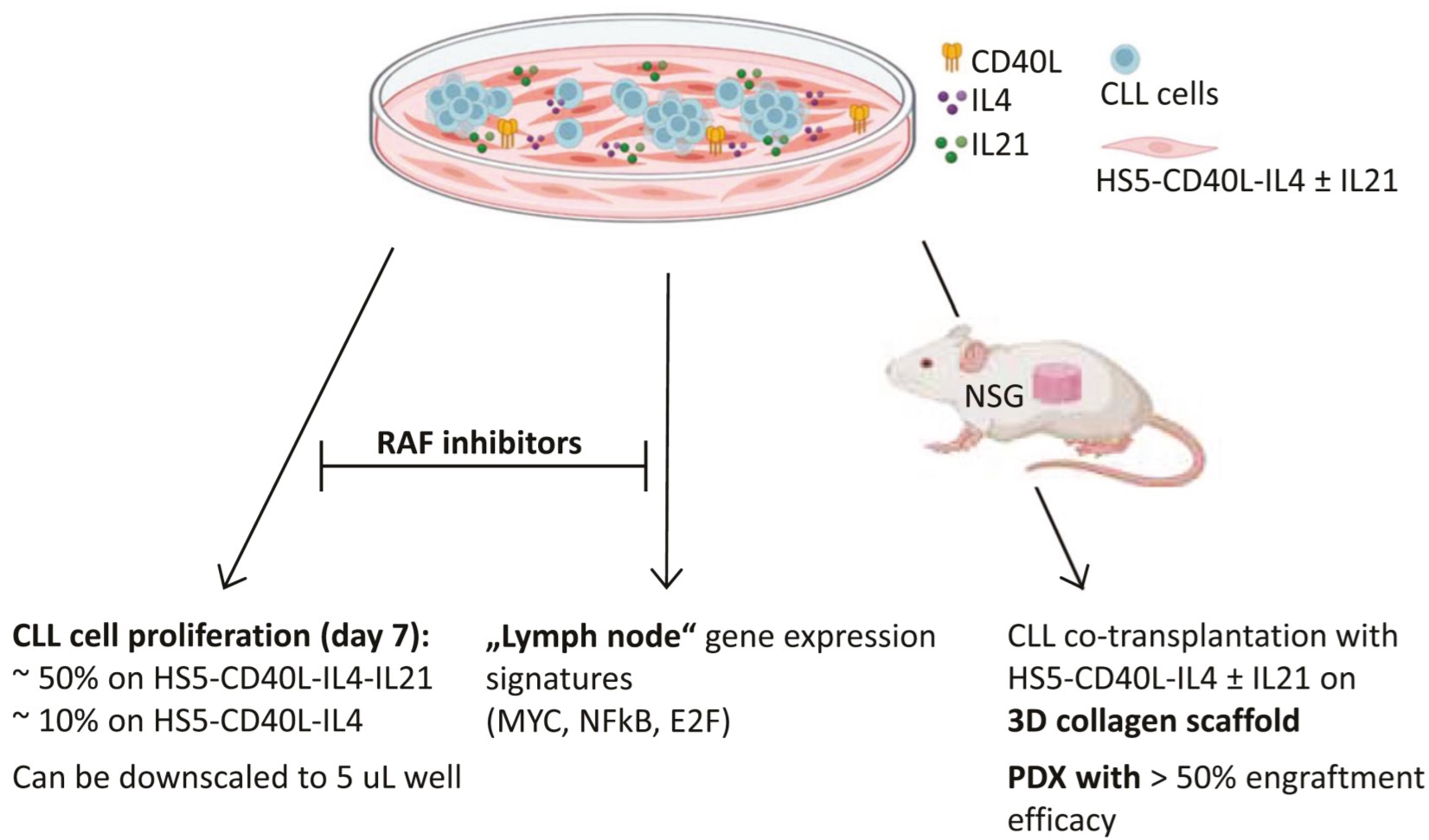
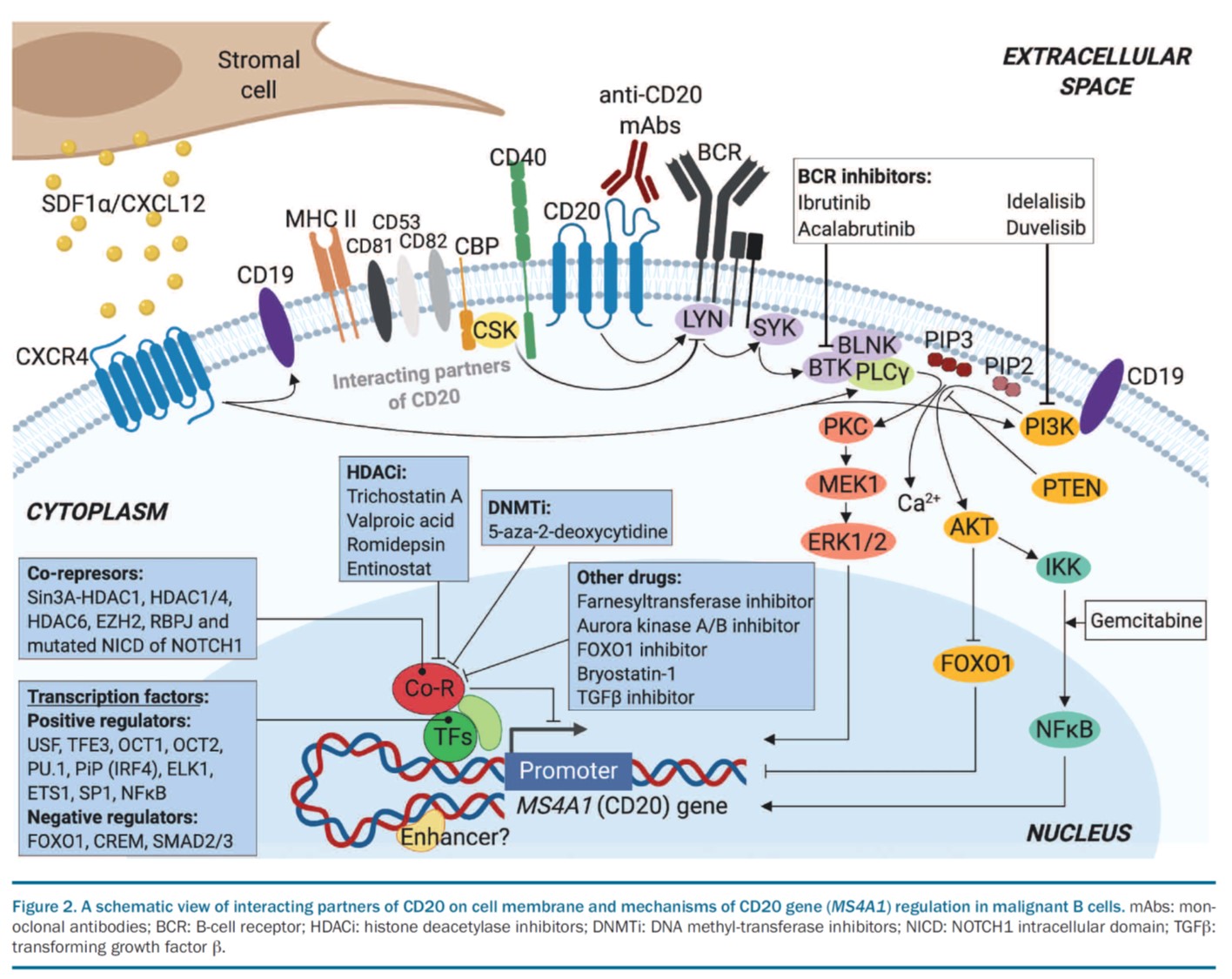
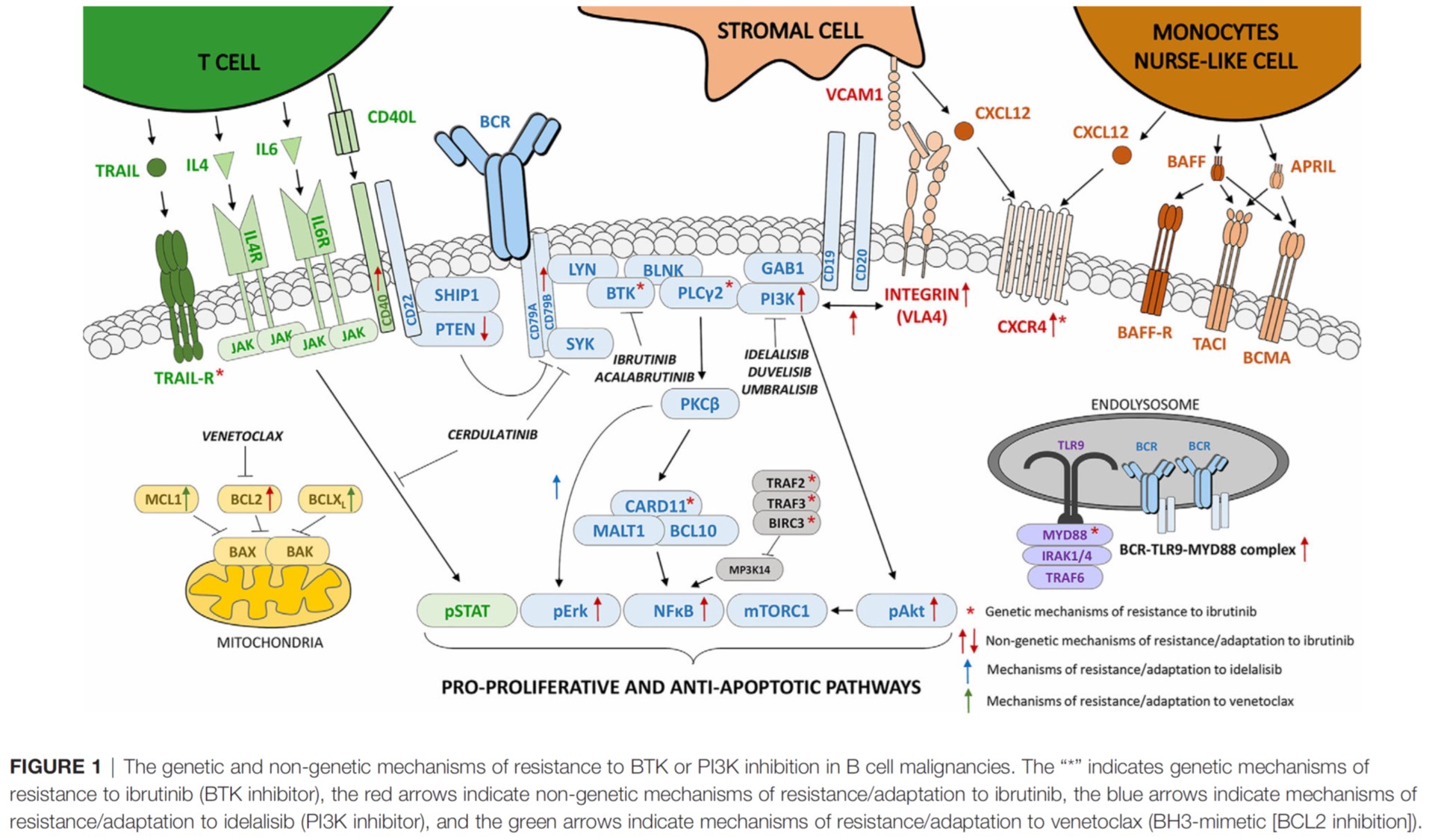
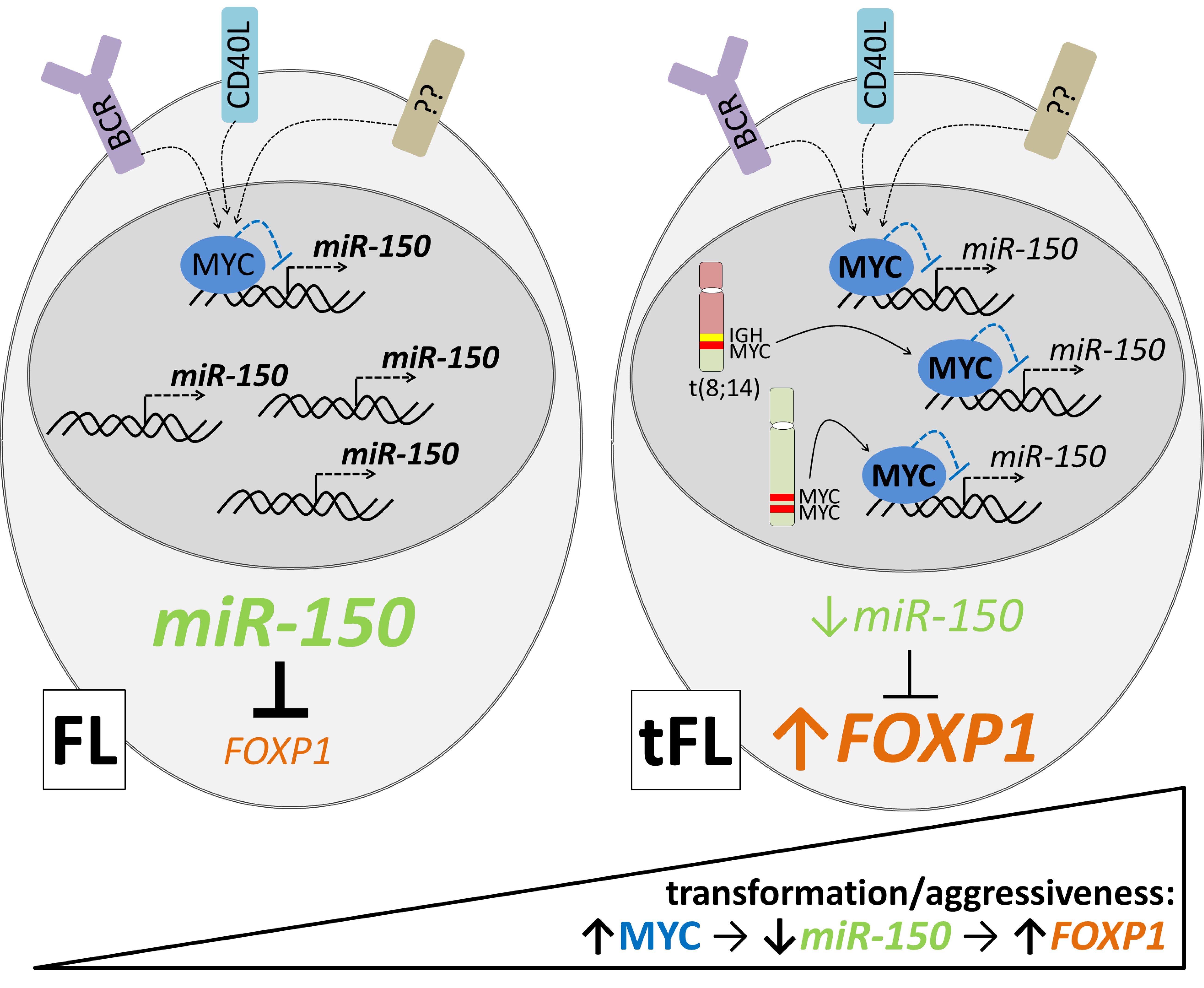
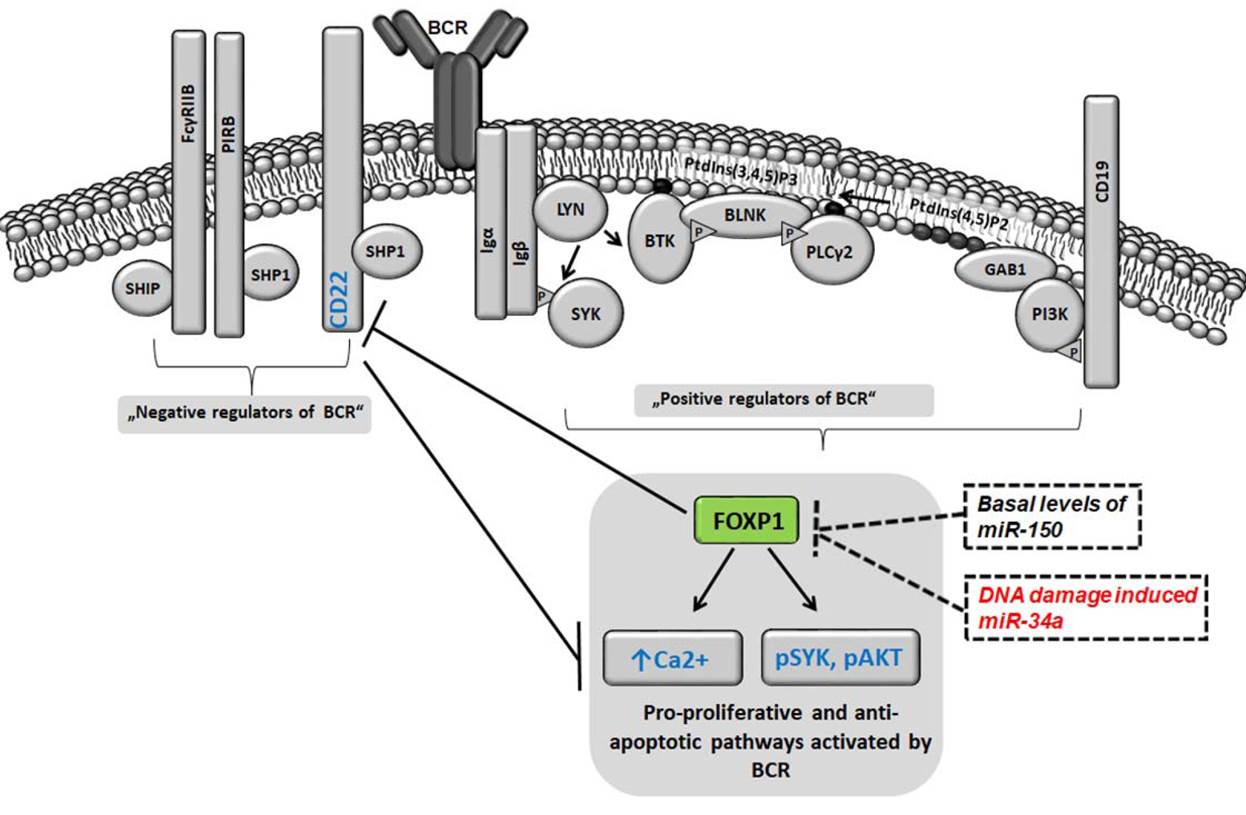
Blood. 2021, IF 25,5.
miR-29 Modulates CD40 Signaling in Chronic Lymphocytic Leukemia by Targeting TRAF4: an Axis Affected by BCR inhibitors
video presenting the research at EHA
awarded as the most influential publication by Czech Hematology Society (2021), and by Masaryk University (MUNI Scientists, Vice-rector award and Deans award for S.Sharma) and CEITEC MU (Award for best publication).
Sharma S,Pavlasova G, Seda V, Cerna K, Vojackova E, Filip D, Ondrisova L, Sandova V, Kostalova L, Zeni PF, Borsky M, Oppelt J, Liskova K, Kren L, Janikova A, Pospisilova S, Fernandes SM, Shehata M , Rassenti LZ, Jaeger U, Doubek M, Davids MS, Brown JR, Mayer J, Kipps TJ,
Mraz M.
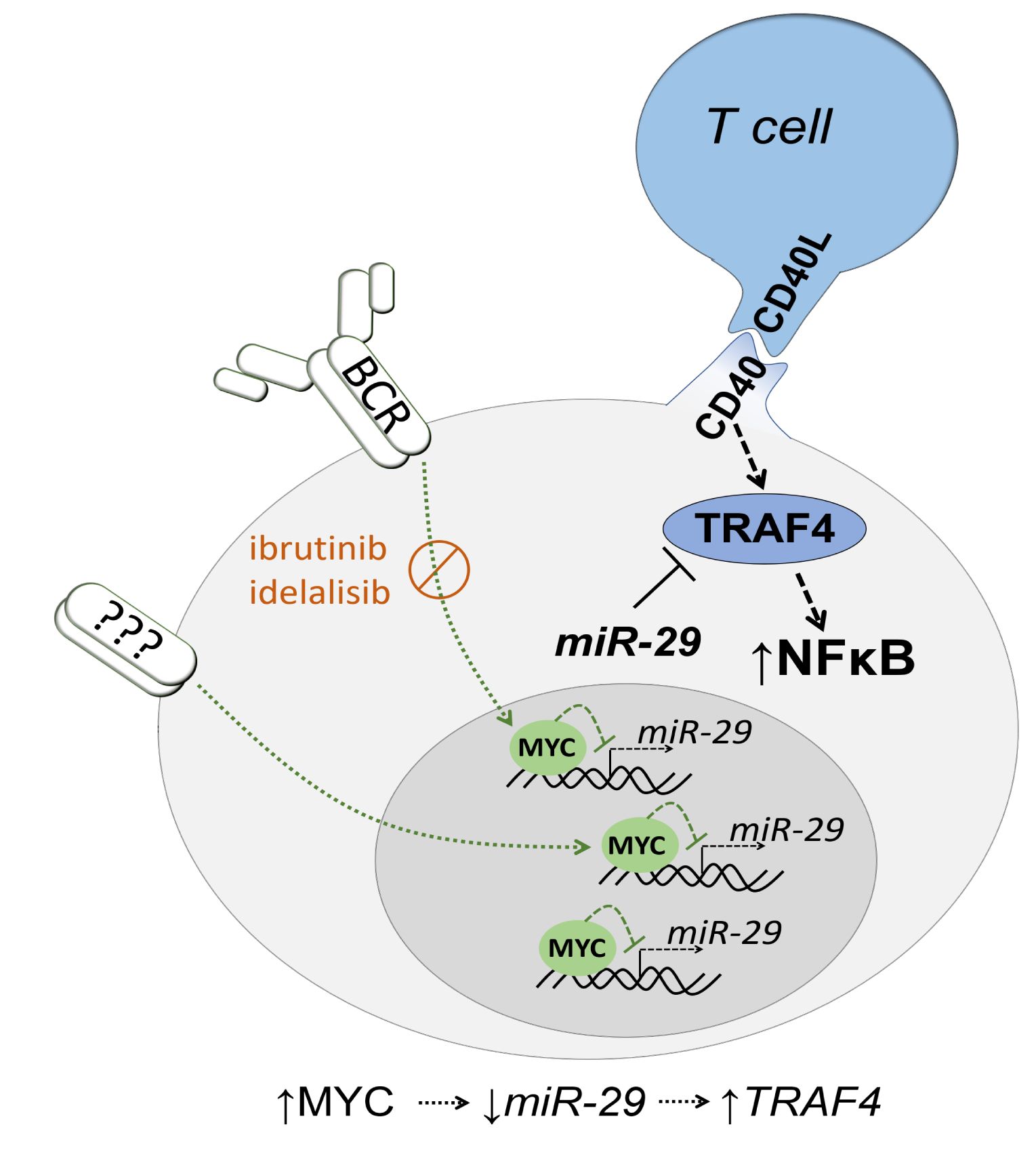
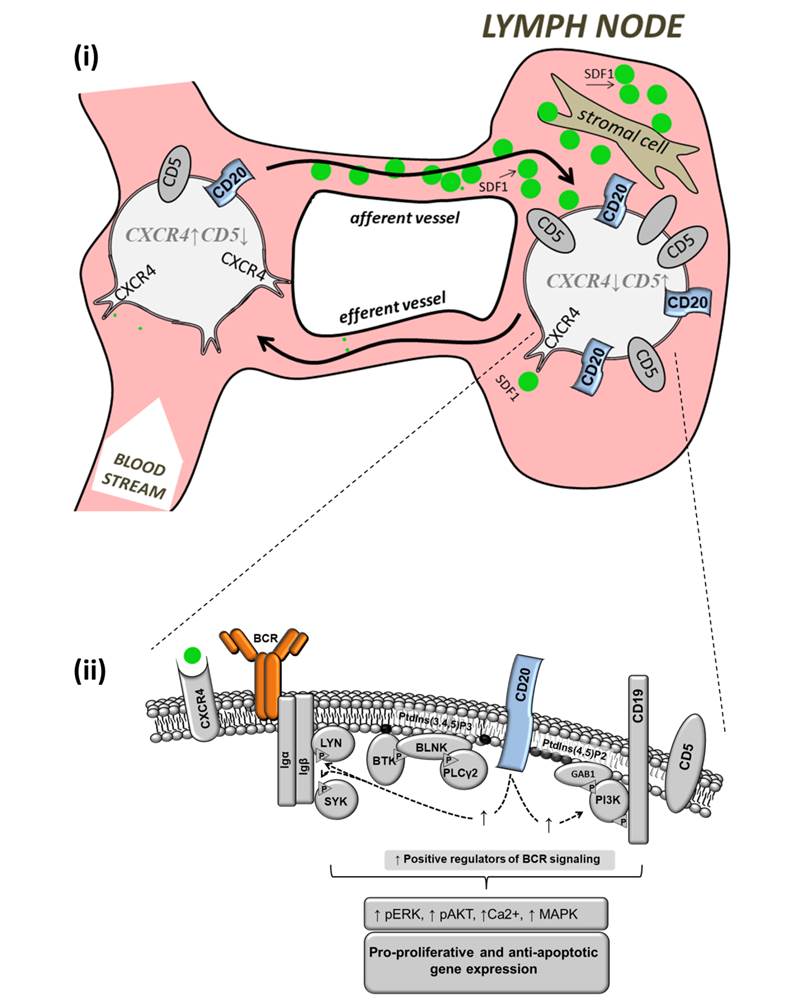
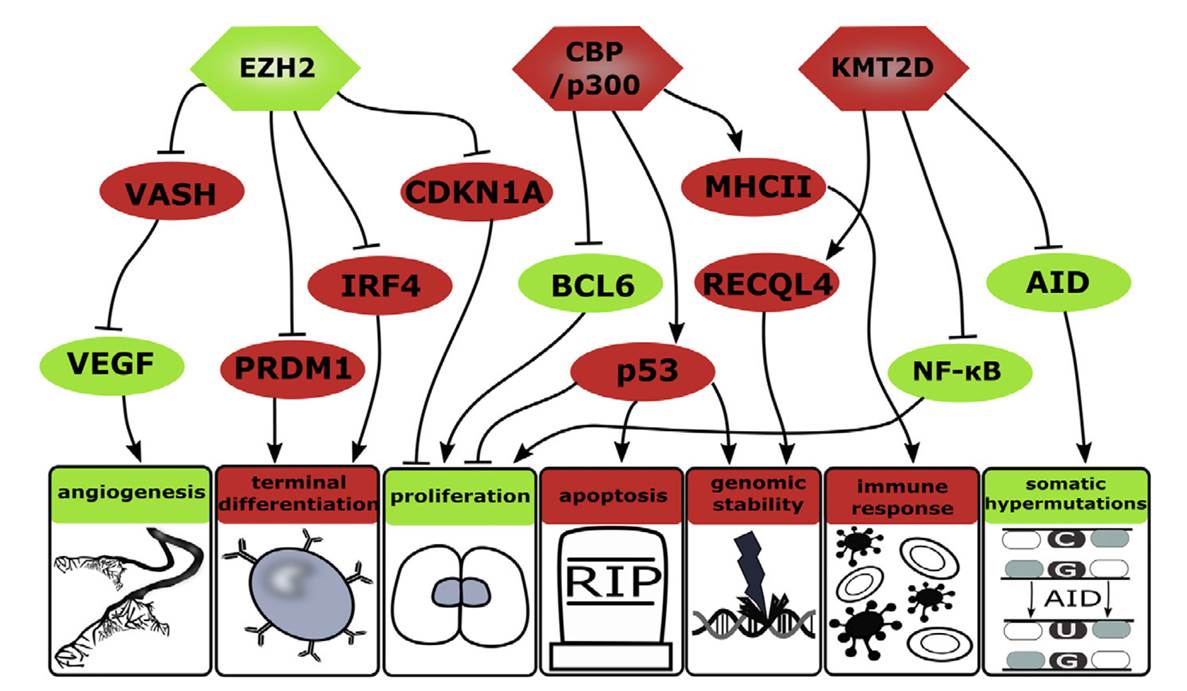
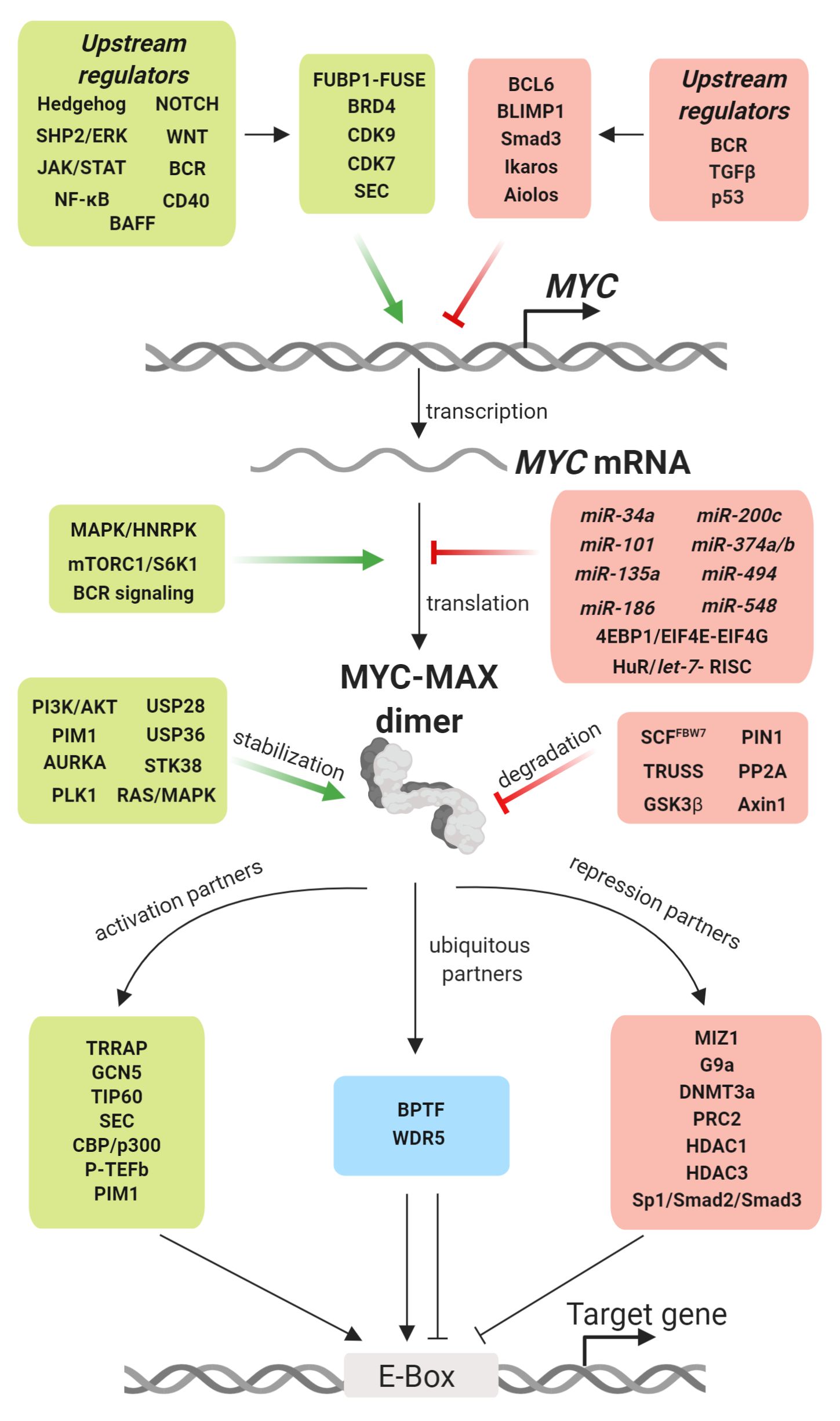
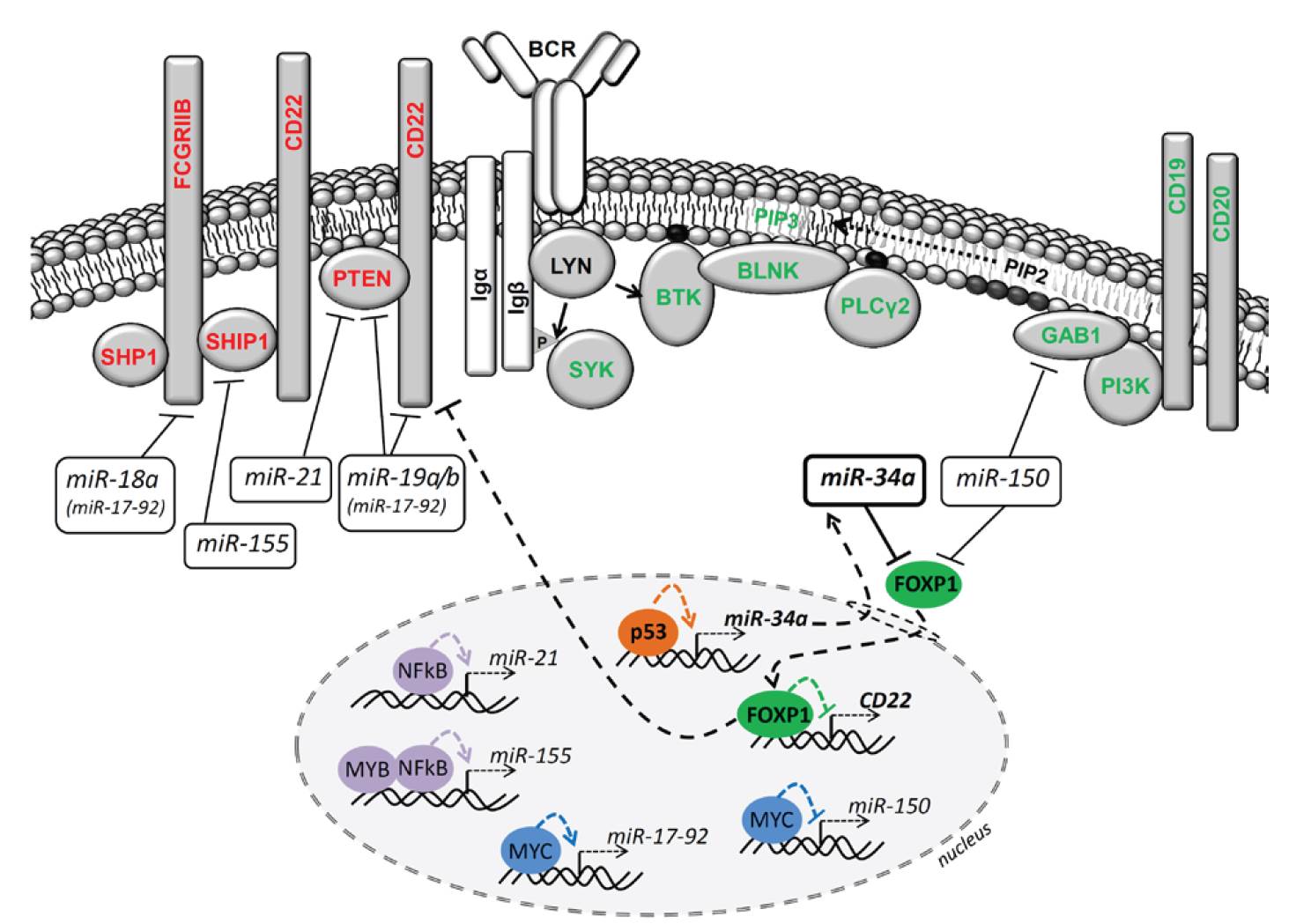
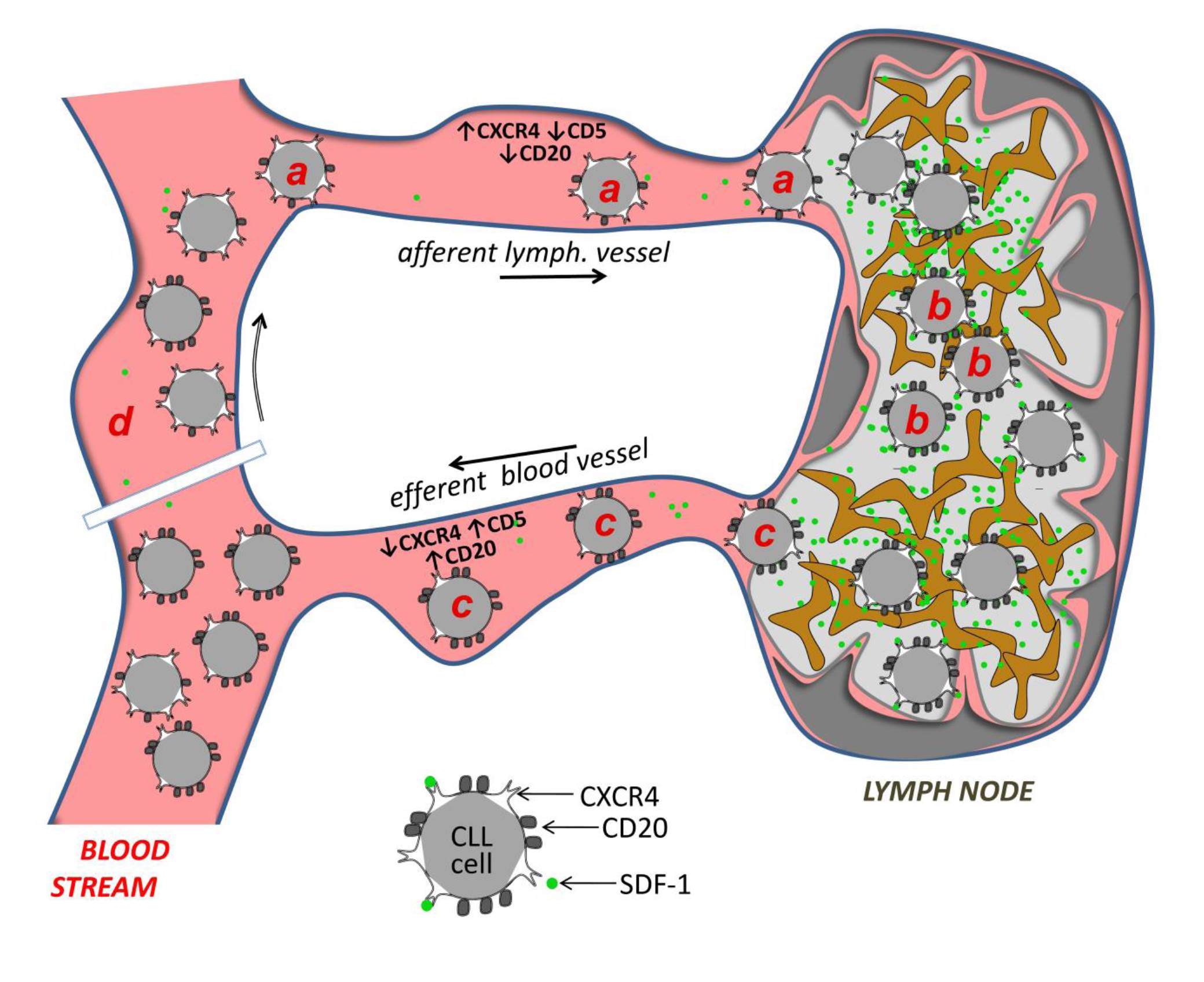
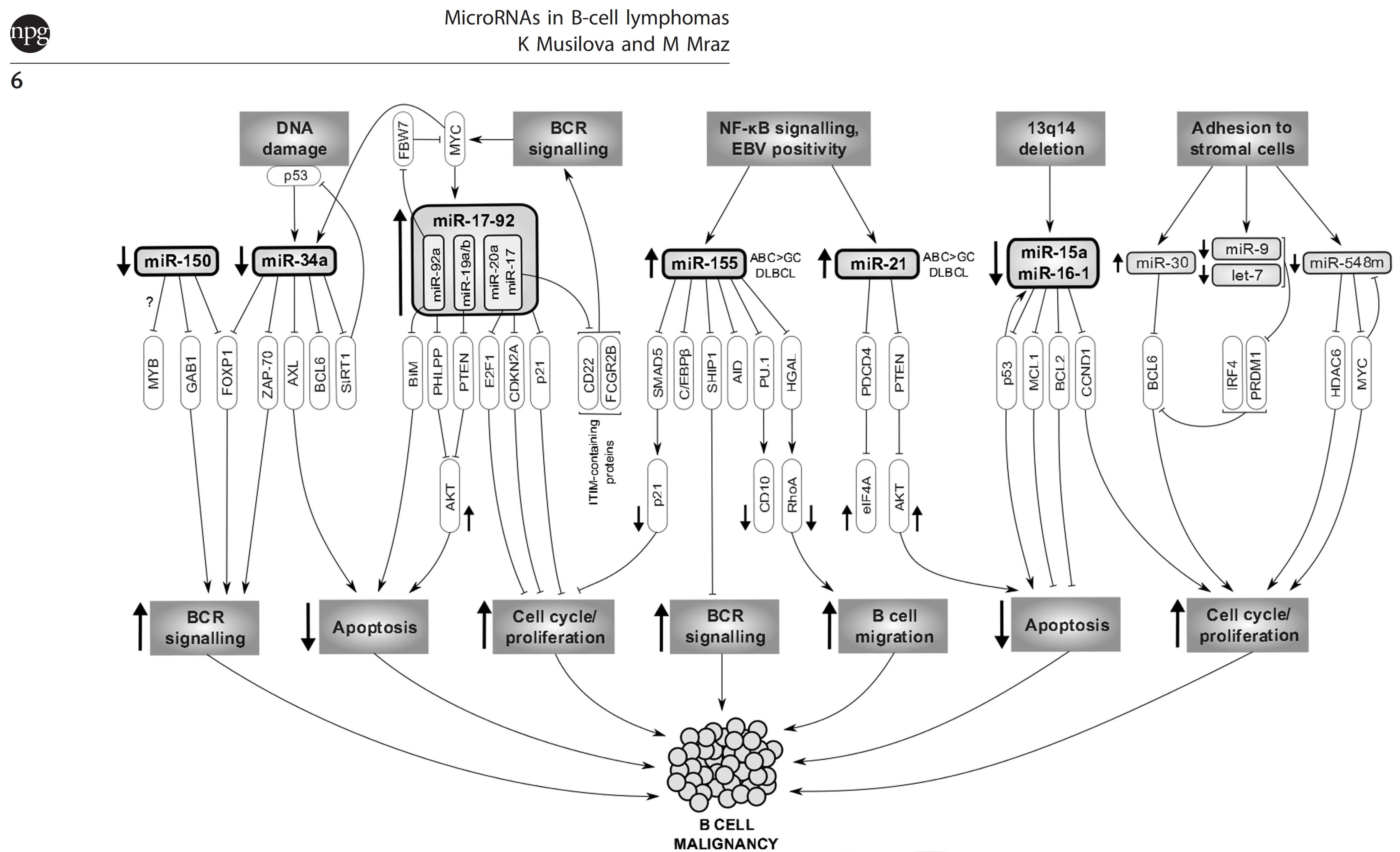
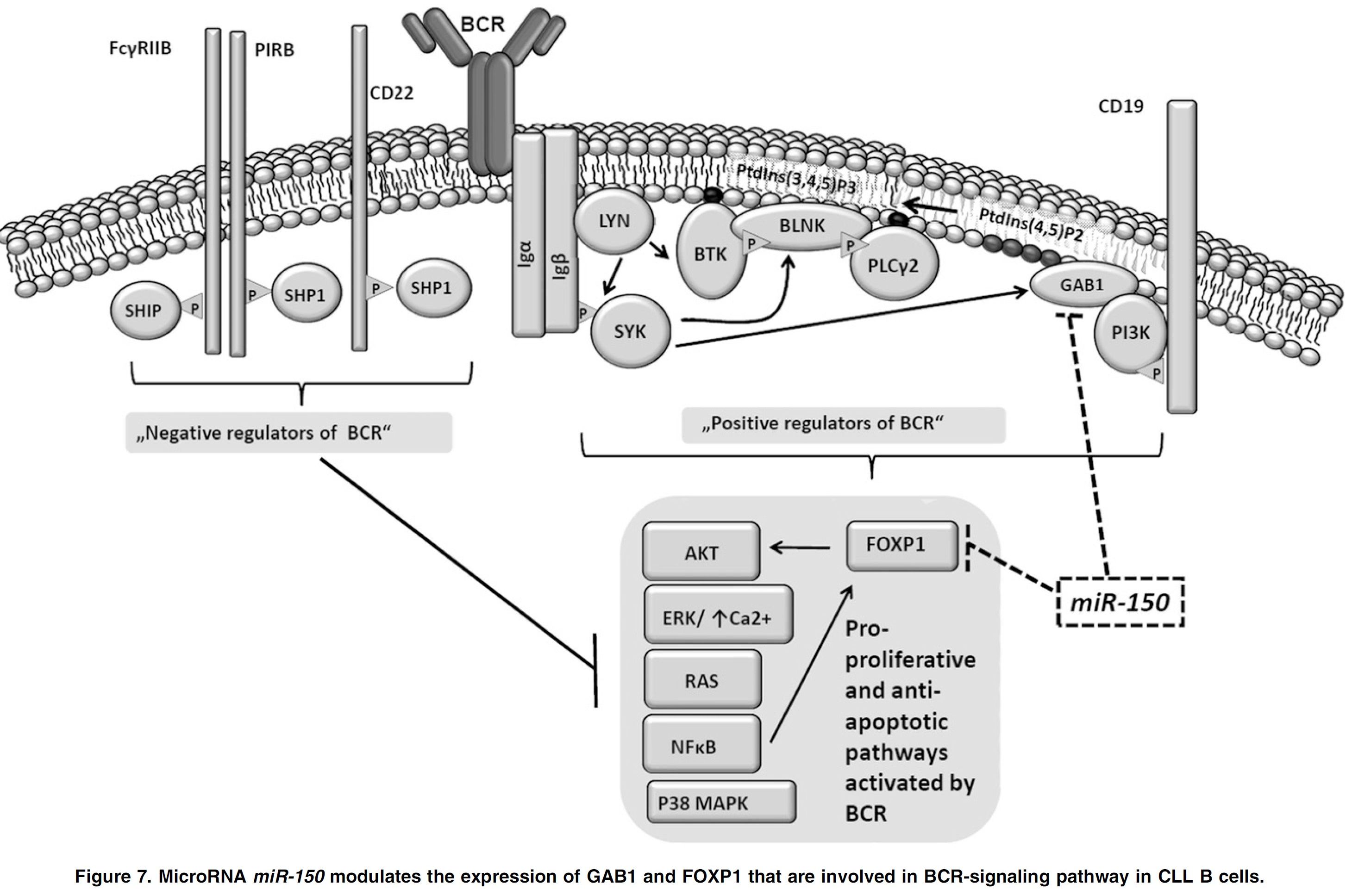
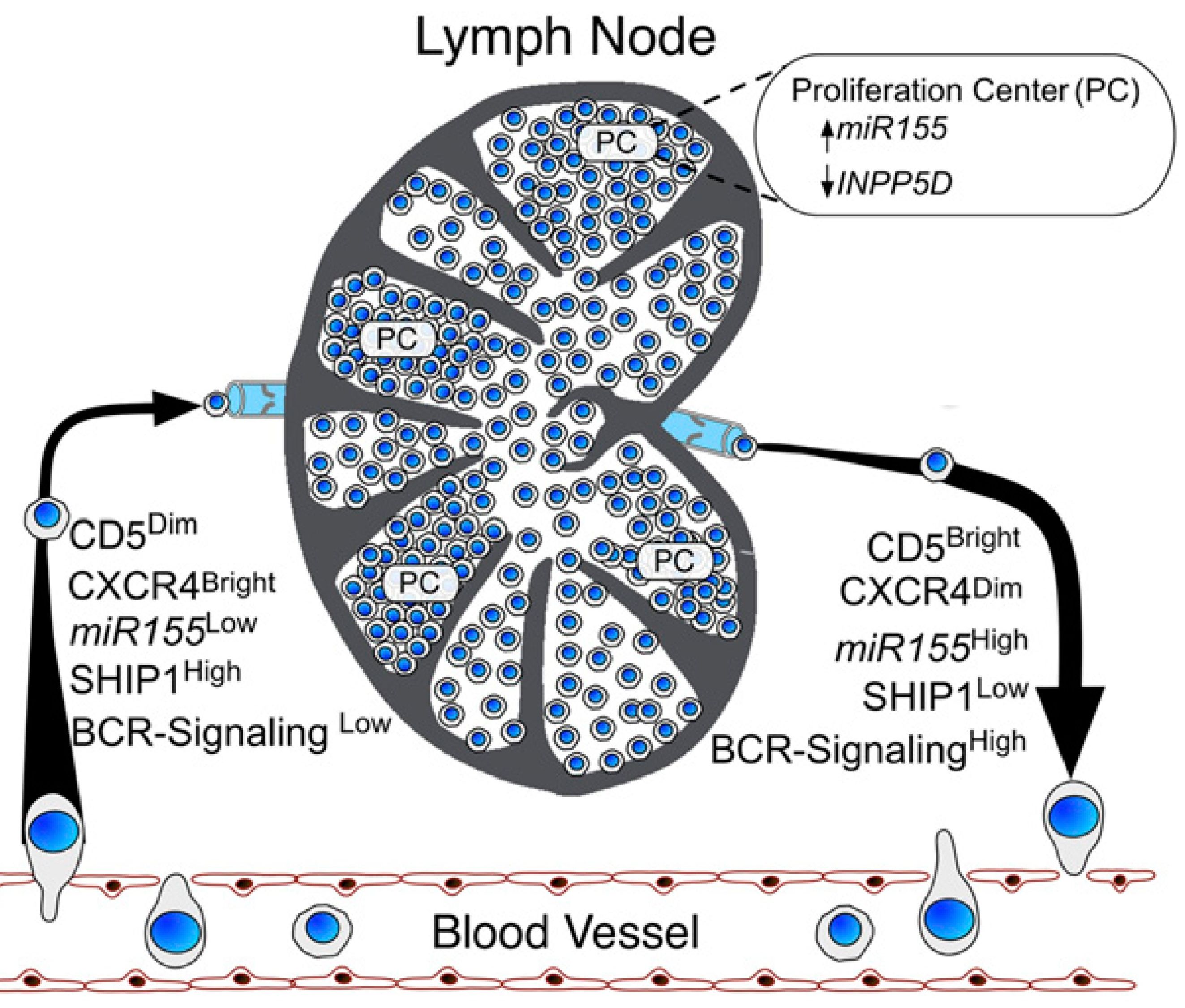
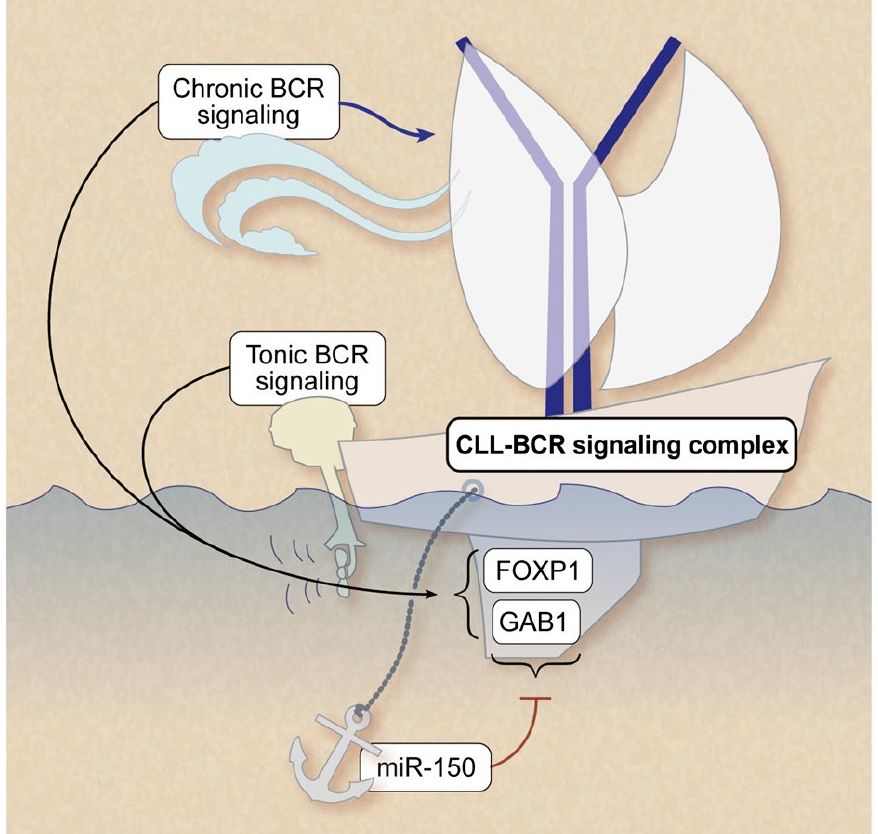
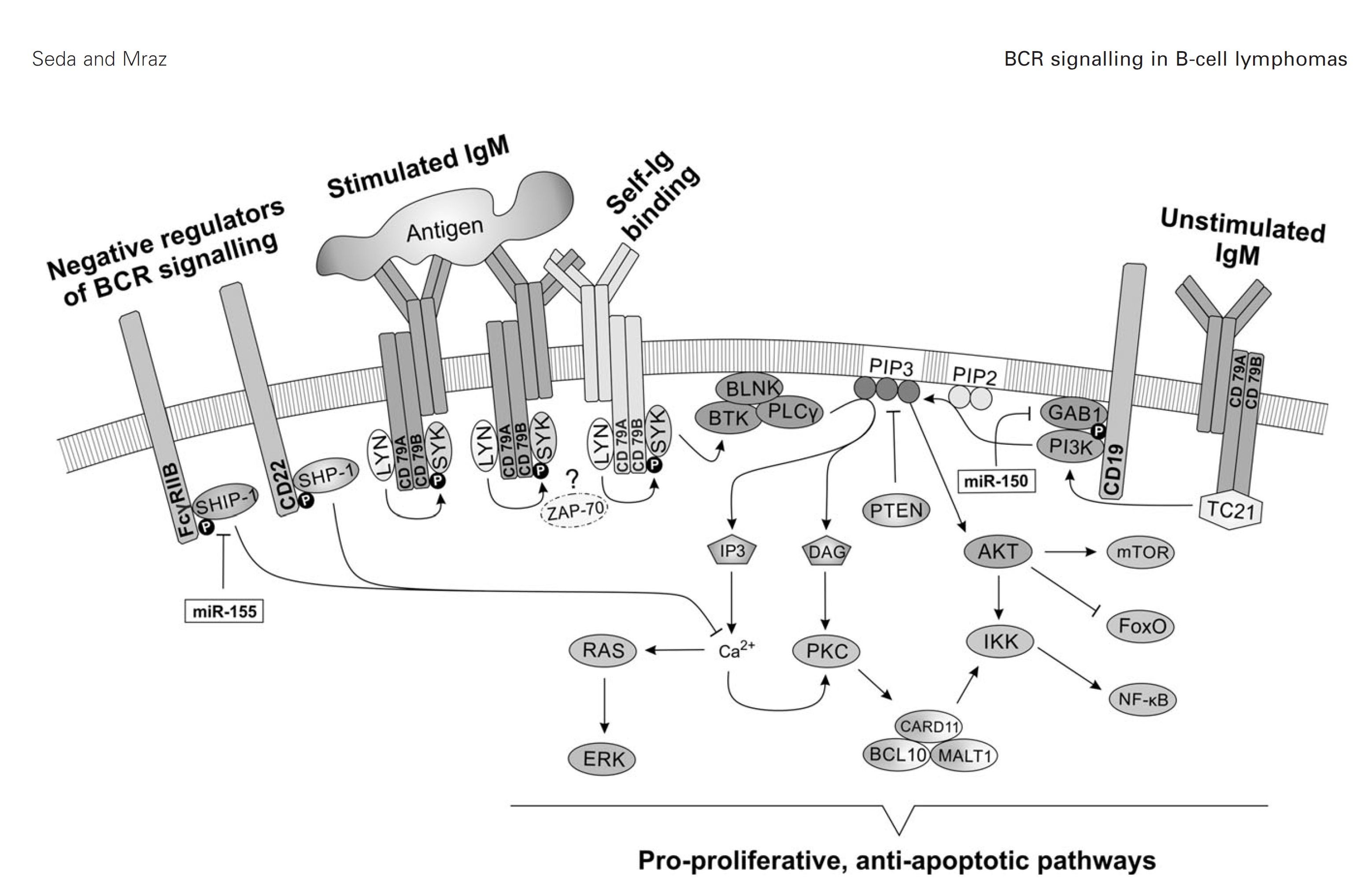
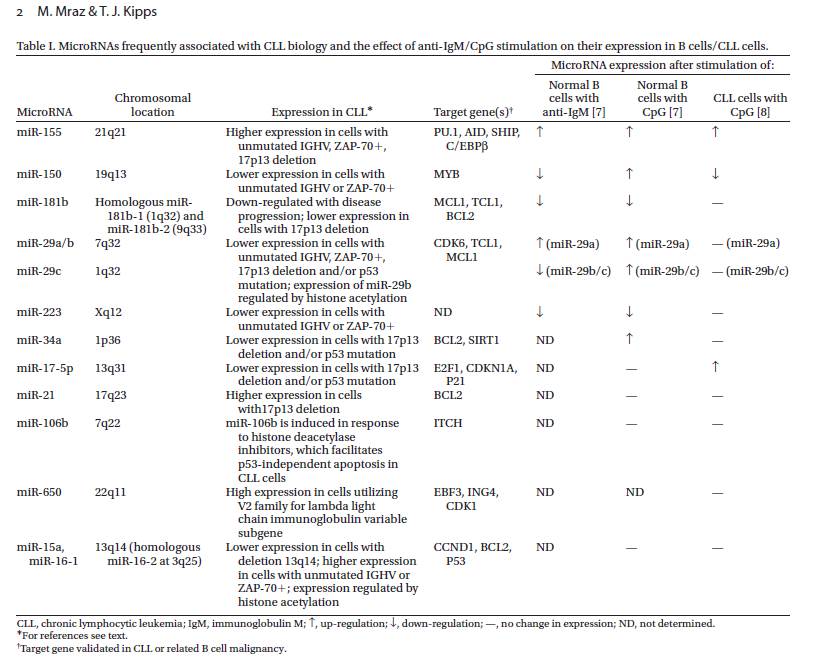
B cell receptor (BCR) signaling and T cell interactions play a pivotal role in chronic lymphocytic leukemia (CLL) pathogenesis and disease aggressiveness. CLL cells can utilize microRNAs (miRNAs) and their targets to modulate microenvironmental interactions in the lymph node niches. To identify miRNA expression changes in the CLL microenvironment, we performed complex profiling of short non-coding RNAs in this context by comparing CXCR4/CD5 intraclonal cell subpopulations (CXCR4dimCD5bright vs. CXCR4brightCD5dim cells). This identified dozens of differentially expressed miRNAs including several that have previously been shown to modulate BCR signaling (miR-155, miR-150, and miR-22), but also other candidates for a role in microenvironmental interactions. Notably, all three miR-29 family members (miR-29a, miR-29b, miR-29c) were consistently down-modulated in the immune niches, and lower miR-29(a/b/c) levels associated with an increased relative responsiveness of CLL cells to BCR ligation, and significantly shorter overall survival of CLL patients. We identified Tumor-Necrosis Factor Receptor-Associated Factor 4 (TRAF4) as a novel direct target of miR-29s and revealed that higher TRAF4 levels increase CLL responsiveness to CD40 activation and downstream NFkB signaling. In CLL, BCR-represses miR-29 expression via MYC, allowing for concurrent TRAF4 upregulation and stronger CD40-NFkB signaling. This regulatory loop is disrupted by "BCR inhibitors" (BTK inhibitor ibrutinib or PI3K inhibitor idelalisib). In summary, we showed for the first time that a miRNA-dependent mechanism acts to activate CD40 signaling/T-cell interactions in a CLL microenvironment and described a novel miR-29-TRAF4-CD40 signaling axis modulated by the BCR activity.